Keywords
Frailty, hypertension, frailty index, FRAIL scale
This article is included in the TILDA gateway.
This article is included in the Ageing Populations collection.
Frailty, hypertension, frailty index, FRAIL scale
Following on from the reviewers’ comments on Version 1, we have made a number of changes to the manuscript.
Firstly, the introduction has been shortened to become more concise and to help focus on the research question more. As part of this change, some of our reflection of the existing studies on hypertension management in frail older adults that were originally in the introduction section, have now been moved to the discussion section.
The nomenclature of the four blood pressure/frailty groups have also been changed. In Version 1 the groups were frail/non-frail with BP treated ‘low’ or ‘high’. Instead, in Version 2 we have changed these groups to frail/non-frail with BP treated ‘below threshold’ (BT) or ‘above threshold’ (AT). This change aims to reflect that our study assesses blood pressure in the context of the ESC/ESH guidelines that recommend the lowest end of the target BP range for older adults treated for hypertension is 130/70mmhg. Therefore our study divides participants into groups based on whether they are a) frail or non-frail and b) whether they are above or below this lower end of the treatment target threshold.
As a result of this change in nomenclature, these 4 frailty-BP groups in the manuscript, including in the Figures and Tables, are now described as either frail AT, frail BT, non- frail AT and non-frail BT.
Finally, in the flow diagram in Figure 1 , the description of the participants ‘’treated for hypertension’’ has been changed to ‘’Prescribed/taking anti-hypertensive medications’’ as our study didn’t specify the indication for the anti-hypertensive medications as there may have been indicatons other than hypertension for these medications being prescribed.
No changes have been made to the original the statistical analyses of Version 1.
See the authors' detailed response to the review by Jane A. H. Masoli
See the authors' detailed response to the review by Nicholas M Pajewski
The management of arterial hypertension in adults has several internationally recognised guidelines for blood pressure (BP) treatment target range. For adults aged ≥65 years, the 2018 European Society of Cardiology / European Society of Hypertension (ESC/ESH) guidelines recommend an office systolic BP treatment range of 130–139 mmHg and a diastolic BP of 70–79 mmHg if tolerated1; and if not tolerated, a higher BP may have to be accepted. ESC/ESH recommend monitoring frail patients closely for side effects of BP lowering medication and in particular to monitor for postural hypotension1. Frailty is a complex state of impaired regulation of physiological systems, which combined with increased vulnerability to stressors can result in adverse health outcomes2. Frailty can result in reduced physiological control of blood pressure3 and impaired cerebral autoregulation4 and has also been proven to be associated with increased risk of cardiovascular morbidity such as stroke and myocardial infarction, and also with cardiovascular mortality5. Therefore, intuitively by controlling and managing cardiovascular risk factors such as hypertension in frail older adults, the risk of adverse cardiovascular health outcomes should be reduced. However, the available evidence would suggest that the interaction between frailty and hypertension management in older adults is more complex and nuanced, as treating hypertension in older adults may have unintended consequences such as orthostatic hypotension6.
When assessing older adults with hypertension, a limitation of the current ESC/ESH guidelines is that no frailty identification tool is specifically advised that would alert clinicians towards possible heightened risk of adverse health outcomes from more intensive BP control. Many clinicians regard the identification of frailty as rather subjective and prone to bias7. If there was a recommended frailty identification method, this would, in theory, inform the clinician as to which patients may require more lenient BP treatment versus those who might tolerate more intensive treatment
Our previous companion study8 demonstrated in a prospective, observational study design that frail older adults, identified as such by the frailty phenotype (FP) and whose BP was treated below ESC/ESH treatment thresholds (BT), had an increased risk of hospitalisation at 2 years. However, in the same study, the frail by Clinical Frailty Scale (CFS) with BP treated BT did not have an increased risk of any of the adverse health outcomes considered. Neither CFS nor FP captured increased risk of falls or fractures, syncope, transient ischaemic attack or stroke, heart attack, heart failure, or mortality at 2 years. However, FP and CFS are only two of the many frailty identification tools available9. Following on from our previous study, in this companion study we aimed to re-analyse the same population cohort but apply two alternative frailty identification tools (Frailty Index and FRAIL scale) to see how they were associated to the same 2-year adverse health outcomes in those below the current recommended BP treatment threshold, as defined by the ESC/ESH guidelines.
For each wave of TILDA, ethical approval was obtained from the Faculty of Health Sciences Research Ethics Committee in Trinity College Dublin: Wave 1 The Irish Longitudinal Study on Ageing (granted on 2nd May 2008) and Wave 2 The Irish Longitudinal Study on Ageing (granted on October 19th 2011). All participants provided written informed consent to participate in TILDA and all experimental procedures complied with the Declaration of Helsinki. Participants had the option to decline to take part or leave the study at any time.
We analysed data from Wave 1 (W1) and Wave 2 (W2) of The Irish Longitudinal Study on Ageing (TILDA). TILDA is a longitudinal cohort study of the health, economic and social conditions of 8,504 community-living adults aged 50 years or over in Ireland. W1 took place between October 2009 and February 2011 and W2 between February 2012 and March 2013. The design and methodology of TILDA and full cohort profile has been documented elsewhere10,11. The W1 assessments comprised of participants engaging in a self-completion questionnaire (SCQ), a computer assisted personal interview (CAPI) and a health assessment performed by trained research nurses. In W2, the participants underwent a repeat SCQ and CAPI. For the purposes of this study, we focused on W1 and W2 only.
At Wave 1, we reviewed the following participant characteristics:
Participant on pharmacological treatment for hypertension and taking any of the prescribed drugs from the following Anatomical Therapeutic Chemical (ATC) Classification System codes (https://www.whocc.no/atc_ddd_index/): 1. C02 (anti-hypertensives), 2. C03 (diuretics), 3. C07 (beta blocking agents), 4. C08 (calcium channel blockers) or 5. C09 (agents acting on the renin-angiotensin system).
Frail by Morley’s five-item FRAIL scale questionnaire (Fatigue, Resistance, Ambulation, Illness & Loss of weight): frailty was defined by the presence of three or more of these criteria12. FRAIL was previously operationalised in TILDA13. Fatigue was scored as 1 point if the participant reported feeling tired “most of the time” or “all of the time” in the previous four weeks. The participants scored 1 point for Resistance if difficulty was reported with walking up 10 steps on stairs without resting and without aids. For Ambulation, the participants scored 1 point if they reported difficulty walking several hundred yards alone without aids. For Illness, the participant scored 1 point if they had five or more of 11 pre-selected illnesses (hypertension, diabetes, cancer other than minor skin cancer, chronic lung disease, heart attack, congestive cardiac failure, angina, asthma, arthritis, stroke and kidney disease). Finally, Loss of weight was scored as 1 point if the participant had a 5% or more weight decline in the preceding 12 months. Participants scoring two or less for these five criteria were identified as non-frail.
Frail by a self-reported 32-item Frailty Index (FI-32). The FI methodology was previously proposed by Rockwood and colleagues14. FI-32 was previously operationalised in TILDA13. Participants were identified as frail with a FI-32 score of ≥ 0.25, which is in keeping with existing studies15. The individual components of this 32-item FI are available in the Extended data16.
Blood pressure (BP) reading: two blood pressure measurements were recorded in the W1 health assessment for each participant. The methodology for measuring BP was standardised with the participant in the upright seated position and two BP measurements taken one minute apart. The OMRONTM digital automatic blood pressure monitor (MODEL M10-IT) with arm cuff was used for BP measurement. The average of the two BP readings was calculated and this reading was then used as the reference blood pressure for each participant. Intensive or ‘low’ blood pressure was defined as SBP < 130 mmHg and/or DBP < 70 mmHg. ‘High’ BP was defined as SBP ≥ 130 mmHg and/or DBP ≥ 70 mmHg.
All Wave 1 participants who were both 65 years or older and had a self-reported history of hypertension that was treated with anti-hypertensive medication were included in the study. Frailty was operationalised as a dichotomous variable for FI and FRAIL as outlined above. Similarly, BP for a participant was dichotomous (BP treated ‘above threshold’ (AT) or BP treated‘below threshold’ (BT)) as per the criteria outlined above. From this BP and frailty data, eight individual baseline Wave 1 BP/frailty groups were then formulated based on a participants frailty status by FI or FRAIL and if their BP was treated ‘below threshold’ or ‘above threshold’.
In keeping with the methodology of our previous study8, the same Wave 2 outcomes were analysed:
• The occurrence of any new falls or fractures (hip, spine, wrist or other) since their first interview at Wave 1.
• The occurrence of a syncopal event, defined as having a faint or blackout, since the first interview.
• Any admission to hospital since Wave 1.
• Any new stroke/TIA, heart failure and heart attack that has been diagnosed or occurred since their first health assessment in Wave 1.
• Death that occurred since the initial Wave 1 assessment.
Statistical analyses were conducted using IBM SPSS Statistics for Windows (version 26.0. Armonk, NY: IBM Corp.). Descriptive statistics were provided as mean with standard deviation (SD), median with interquartile range (IQR) or number (N) with percentage (%). For the assessment of differences between the eight baseline BP/frailty groups formulated in Wave 1, the Chi-squared test was used for binary variables and the Kruskal-Wallis test for continuous variables.
Binary logistic regression models were used to assess the independent association between baseline Wave 1 BP/frailty groups and the Wave 2 longitudinal outcomes. The two differently adjusted models were utilised each time as follows:
1. Basic logistic regression model – adjusted for age and sex only.
2. Full logistic regression model – adjusted for age, sex, and all the following Wave 1 characteristics:
Orthostatic hypotension (OH), which for the purposes of this study we defined as a drop of ≥20 mmHg in SBP and/or ≥10 mmHg DBP on standing upright from a seated position. OH was measured in TILDA and described in detail elsehwere17.
Polypharmacy, which for this study was present if a participant was taking ≥ 5 regular prescribed medications.
Lower educational attainment (up to primary school level).
Cognition as determined by the Montreal Cognitive Assessment (MOCA) score18
Microsoft Excel version 2203 (Build 15028.20228) was used to generate forest plots in order to display the adjusted odds ratio (OR) for each of the BP/frailty groups and the longitudinal outcomes assessed at Wave 2. The 95% confidence interval (CI) for each OR and P value were also included in the forest plots. Statistical significance was set at P<0.05 throughout.
In Wave 1 of TILDA, there was a total of 1,920 participants aged ≥65 years who were on medication to treat hypertension. Of these, 1,274 participants had complete FI/BP data and 1,276 complete FRAIL/BP data. Approximately one in three participants were frail by FI (n=429, 33.7%), while only 5.5% (n=70) were frail by FRAIL scale. Figure 1 summarises how the final frailty/BP groups were arrived at.
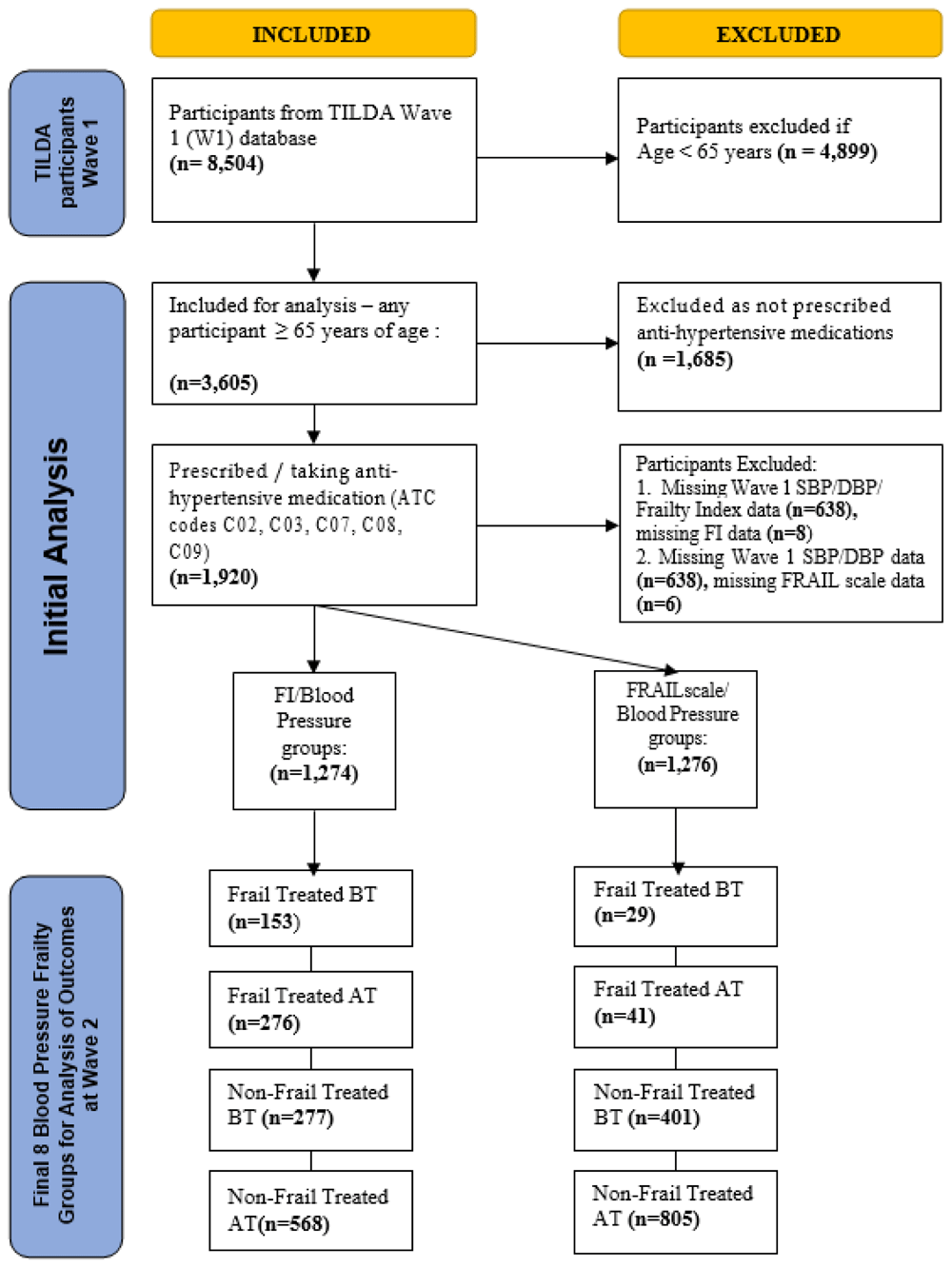
FI = Frailty Index, FRAIL scale (Fatigue, Resistance, Ambulation, Illnesses and Resistance), SBP = systolic blood pressure, DBP = diastolic blood pressure, BT = Blood pressure treated below 130/80mmHG threshold, AT= Blood pressure treated above/equal to the 130/80mmHG threshold.
The baseline characteristics of both FI/BP and FRAIL/BP groups are summarised in Table 1 and Table 2, respectively. The frail by FI with BP treated low or high at Wave 1 seemed older (p<0.001), more likely to be of female sex (p<0.001), have a higher proportion of lower education (p=0.008), be more comorbid and disabled (both p<0.001), have lower MOCA scores (p<0.001), and have more frequent polypharmacy (p<0.001) (Table 1). For the frail versus non-frail by FRAIL scale at baseline, there was no significant difference in age or sex, but the frail also seemed more comorbid and disabled (both p<0.001), have lower MOCA scores (p<0.001), and have more frequent polypharmacy (p<0.001).
Legend – SD – standard deviation, IQR – interquartile range, SBP – systolic blood pressure, DBP – diastolic blood pressure, MOCA – Montreal Cognitive Assessment score.
| Frail Treated Below Threshold n= 153 | Frail Treated Above Threshold n= 276 | Non-Frail Treated Below Threshold n= 277 | Non-Frail Treated Above Threshold n=568 | P Value (overall difference) | |
|---|---|---|---|---|---|
| Wave 1 characteristics | |||||
| Mean Age (SD) | 75.1 (6.3) | 75.8 (6.8) | 73.0 (5.9) | 72.9 (6.3) | <0.001* |
| Female Sex (%) | 95 (62.1) | 161 (58.3) | 123 (44.4) | 246 (43.3) | <0.001# |
| Education level: up to primary only (%) | 74 (48.4) | 138 (50) | 114 (41.2) | 220 (38.7) | 0.008# |
| Median number of chronic diseases (IQR) | 4 (2) | 4 (2) | 2 (1) | 2 (2) | <0.001* |
| Median number of physical limitations (IQR) | 6 (3) | 6 (3) | 2 (2) | 1 (2) | <0.001* |
| Median MOCA score (IQR) | 22 (7) | 22.12 (6) | 24 (5) | 25 (3.92) | <0.001* |
| Polypharmacy (%) | 125 (81.7) | 210 (76.1) | 135 ( 48.7) | 201 (35.4) | <0.001# |
| Mean seated SBP mmHg (SD) | 119.5 (12.6) | 152.9 (18.3) | 122.4 (11.1) | 151.4 (16.3) | &0.001* |
| Mean seated DBP mmHg (SD) | 70.9 (12.4) | 90.7 (20.2) | 71.0 (8.8) | 86.0 (11.6) | <0.001* |
| Orthostatic hypotension (%) | 13 (8.5) | 51 (18.8) | 18 (6.5) | 72 (12.7) | <0.001# |
| Wave 2 outcomes | |||||
| Any fall or fracture (%) | 60 (45.5) | 93 (39.9) | 49 (20.0) | 137 (26.0) | <0.001# |
| New syncope (%) | 6 (6.1) | 15 (8.8) | 6 (3.0) | 17 (3.9) | 0.035# |
| New hospitalisation (%) | 48 (36.4) | 72 (30.9) | 50 (20.3) | 104 (19.7) | <0.001# |
| New stroke or TIA (%) | 5 (3.8) | 13 (5.6) | 3 (1.2) | 17 (3.2) | 0.067# |
| New heart failure (%) | 5 (3.8) | 0 (0) | 1 (0.4) | 7 (1.3) | 0.007# |
| New heart attack (%) | 3 (2.6) | 3 (1.4) | 2 (0.9) | 4 (0.8) | 0.416# |
| Mortality (%) | 12 (7.8) | 19 (6.9) | 10 (3.6) | 15 (2.6) | 0.005# |
Legend – SD – standard deviation, IQR – interquartile range, SBP – systolic blood pressure, DBP – diastolic blood pressure, MOCA – Montreal Cognitive Assessment score.
| Frail Treated Below Threshold n= 29 | Frail Treated Above Threshold n= 41 | Non-Frail Treated Below Threshold n= 401 | Non-Frail Treated Above Threshold N= 805 | P Value (overall difference) | |
|---|---|---|---|---|---|
| Wave 1 characteristics | |||||
| Mean Age (SD) | 74.9 (5.7) | 75.6 (7.4) | 73.6 (6.1) | 73.8 (6.) | 0.207* |
| Female Sex (%) | 15 (51.7) | 23 (56.1) | 203 (50.6) | 386 (48.0) | 0.641# |
| Education level: up to primary only (%) | 15 (51.7) | 25 (61.0) | 173 (43.1) | 333 (41.5) | 0.070# |
| Median number of chronic diseases (IQR) | 5 (3) | 5 (3) | 3 (2) | 3 (2) | <0.001* |
| Median number of physical limitations (IQR) | 7 (3) | 7 (3) | 3 (4) | 2 (3) | <0.001* |
| Median MOCA score (IQR) | 20 (6) | 21 (7) | 23 (6) | 24 (6) | <0.001* |
| Polypharmacy (%) | 26 (89.7) | 34 (82.9) | 234 (58.4) | 377 (46.8) | <0.001# |
| Mean seated SBP mmHg (SD) | 118.9 (13.4) | 150.8 (17.6) | 121.6 (11.6) | 151.9 (16.9) | <0.001* |
| Mean seated DBP mmHg (SD) | 68.4 (7.1) | 90.3 (20.2) | 71.1 (10.4) | 87.4 (14.8) | <0.001* |
| Orthostatic hypotension (%) | 1 (3.4) | 5 (13.5) | 30 (7.5) | 118 (14.7) | 0.002# |
| Wave 2 outcomes | |||||
| Any fall or fracture (%) | 13 (59.1) | 14 (43.8) | 96 (27.0) | 216 (29.6) | 0.004# |
| New syncope (%) | 1 (7.7) | 0 (0) | 11 (3.8) | 32 (5.4) | 0.557# |
| New hospitalisation (%) | 13 (59.1) | 14 (43.8) | 85 (23.9) | 162 (22.2) | <0.001# |
| New stroke or TIA (%) | 1 (4.5) | 1 (3.1) | 7 (2.0) | 29 (4.0) | 0.377# |
| New heart failure (%) | 2 (9.1) | 0 (0) | 4 (1.1) | 7 (1.0) | 0.005# |
| New heart attack (%) | 0 (0) | 0 (0) | 5 (1.6) | 7 (1.0) | 0.752# |
| Mortality (%) | 5 (17.2) | 5 (12.2) | 17 (4.2) | 29 (3.6) | <0.001# |
The Wave 2 outcomes of both FI/BP and FRAIL/BP groups are summarised in Table 1 and Table 2, respectively. The results of the basic and full binary logistic regression models for the risk of longitudinal health outcomes at Wave 2 for the eight BP/frailty sub-groups are summarised in Figure 2. The results in Figure 2 are colour coded to highlight groups with a statistically significant increased risk (red), reduced risk (green) or no statistical significance/risk (yellow) for all the individual health outcomes analysed at Wave 2. The full detailed results of the binary logistic regression models are available in the Extended data16.
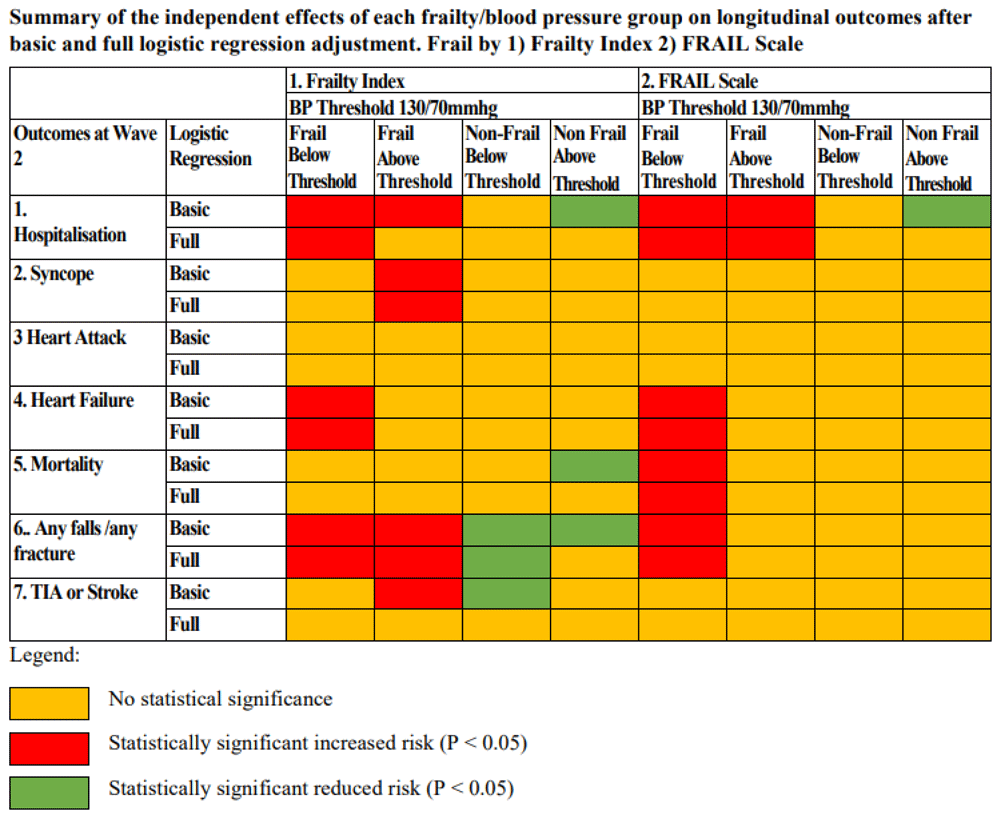
BP = Blood Pressure, FRAIL Scale = Fatigue Resistance Ambulation Illness Loss of weight Scale , TIA or Stroke = Transient Ischaemic Attack or Stroke
In the fully adjusted models, the frail with BP treated below threshold (BT) for both FI and FRAIL were at increased risk of hospitalisation, heart failure and falls/fractures in Wave 2 (FI: hospitalisation P=0.015, heart failure P=0.031, falls/fracture P=0.002; FRAIL: hospitalisation P=0.002, heart failure P=0.012, falls/fracture P=0.007). The frail by FRAIL with BP treated BT were also at increased risk mortality (P=0.019) in the fully adjusted models. The frail by FI treated above threshold (AT) had an increased risk of syncope (P=0.030) and falls/fractures (P=0.006). Frail by FRAIL scale treated AT had increased risk of hospitalisation (P=0.024). The non-frail by FI or FRAIL did not have increased risk of any of the adverse outcomes studied. The non-frail by FI treated BT had a significantly reduced risk of falls/fractures by Wave 2 (P=0.001) in the fully adjusted model.
The forest plots of the fully adjusted ORs for each of the frailty/BP sub-groups and longitudinal outcomes assessed, with 95% confidence intervals for the ORs and associated P values included, are available in Figure 3(a) and Figure 3(b).
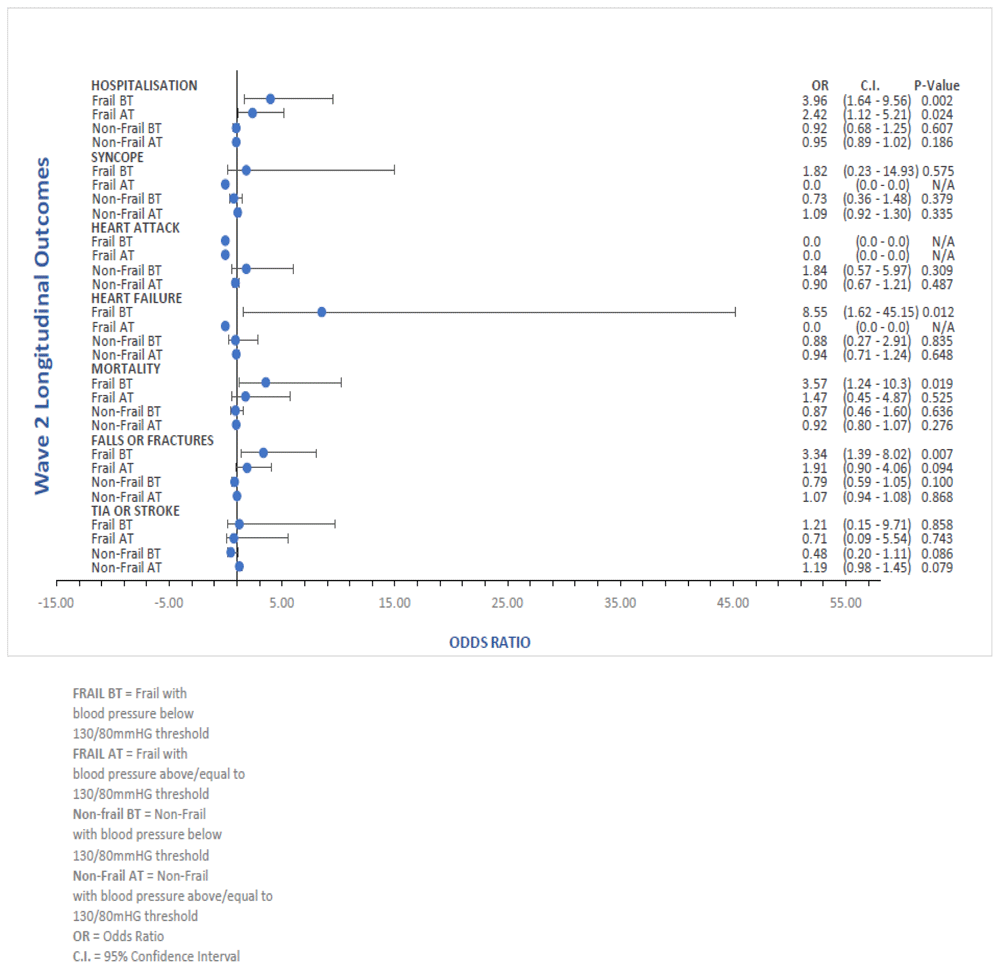
FRAIL Scale = Fatigue Resistance Ambulation Illness Loss of weight Scale, TIA or Stroke = Transient Ischaemic Attack or Stroke, O.R. = Odds Ratio, C.I. = Confidence Interval.
The latest 2018 guidelines from the European Society of Cardiology/European Society of Hypertension (ESC/ESH) for the management of hypertension in adults aged ≥65 years advise a blood pressure (BP) treatment target of 130–139/70–79 mmHg if tolerated by the patient. On the contrary, recent high profile randomised controlled trials have been promoting lower BP targets. The STEP trial identified that intensive BP treatment in older adults to a target of 110–130 mmHg resulted in a lower incidence of cardiovascular events compared to standard treatment, without associated risk of dizziness, syncope or fractures19. Similarly, SPRINT investigators reported a lower all-cause mortality and lower rates of adverse cardiovascular events in older adults treated to a systolic BP of less than 120 mmHg20. A secondary analysis of SPRINT also showed no increased risk of falls in those with intensive blood pressure management compared to standard treatment21. A criticism of both trials is the exclusion of frail older adults such as those with heart failure, advanced cognitive impairment, poorly controlled diabetes or even those who require institutional nursing home care22. Therefore, whilst undoubtedly these RCTs demonstrate the cardiovascular benefits of strict blood pressure control in relatively healthy older adult populations, the generalisability of these results to frail, multi-morbid patients is yet unproven. Therefore, current guidelines state that caution and individualisation of antihypertensive treatment should be applied in the frail.
However, different operationalisations of frailty exist in the literature. Following on from our previous study8, in this companion paper we re-analysed the same population cohort but applied two alternative frailty identification tools (Frailty Index and FRAIL scale) to see how they were associated to the same 2-year adverse health outcomes in those intensively treated for hypertension. Again, we showed that the increased risk of adverse outcomes did not occur in non-frail, but only frail groups. Here, we showed that most adverse health outcomes at 2 years occurred in the frail with BP treated below current ESC/ESH recommended threshold (BT). In this study utilising the FI and the FRAIL scale, we found more adverse outcome associations compared to our previous study with the Frailty Phenotype and the Clinical Frailty Scale. Taken together, combined results further establish that the way frailty is identified is crucial for the application of the ESC/ESH guideline, because different frailty identification tools behave differently in how they capture longitudinal risks.
The fact that again, frailty was seen in association with adverse outcomes reflects existing knowledge and previous studies that identified frailty as a risk factor for falls23, fractures24, mortality25, heart failure26 and hospitalisation27. The evidence regarding potential harm from anti-hypertensive medication is mixed. In the REGARDS prospective population-based cohort study, the authors identified that two or more frailty indicators at baseline were predictive of increased risk of serious fall-related injuries, but there was no increased risk or association with systolic blood pressure (SBP), diastolic blood pressure (DBP) or number of antihypertensive medications. Conversely, Tinetti et al. suggested that anti-hypertensive medications may be associated with increased risk of hypotension, falls and fall-related injuries28.
Within the frail groups of this study, there were some differences in how the FI and FRAIL scale predicted the outcomes assessed. Firstly, for the outcome of syncope by Wave 2, the only group that had increased risk was the frail by FI with BP treated AT. Low blood pressure and over-treated hypertension are well-known risk factors for syncope29,30, so a possibility (that we cannot verify with our design) is that some participants in this group may have been previously identified by their medical practitioners as having increased cardiovascular risk and had their anti-hypertensive medications reduced or de-prescribed. The FI incorporates more cardiovascular co-morbidities than the FRAIL scale and this may explain why this finding was not captured in the frail by FRAIL scale treated AT. On the other hand, the prevalence of frail by FI was greater than that of FRAIL scale, which confers greater statistical power for the FI analyses.
Both FI and FRAIL identified an increased risk of a new diagnosis of heart failure by Wave 2 for the frail treated BT. The FI incorporates multiple risk factors for cardiovascular disease and heart failure including hypertension, angina, heart attack, diabetes and high cholesterol. In this light, a higher risk of new heart failure would be in keeping with the cumulative deficits model of frailty31. In addition, while heart failure is not a specific item of the FI, one of the deficits included in the 32-item FI was “other cardiovascular disease’’, which could include heart failure. For the frail by FRAIL treated BT, a different mechanism of how heart failure is captured is possible. The FRAIL scale incorporates fatigue as one of its criteria and fatigue is also a well-recognised clinical symptom in patients with chronic heart failure32,33. As outlined in our companion paper8, frail by FP (which also includes fatigue) treated BT also captured increased heart failure risk. However, this consistency across the FP, FI and FRAIL with BP treated BT in association with new heart failure could also be explained by reduced ejection fraction (‘pump failure’) causing low BP that may still require long term cardiovascular medications to reduce morbidity and mortality.
The frail by FRAIL scale treated BT had an increased risk of mortality by Wave 2. This is in keeping with an existing study on the TILDA cohort where the FRAIL scale was identified as the most specific frailty identification tool for the prediction of 8-year mortality in comparison with FP, FI and CFS34. In the same study, the FI had the lowest specificity for predicting mortality and this is in keeping with our study where the FI did not capture any increased risk of short-term mortality for the frail treated low or high. The systolic BP decline in the final 2 years of life, in patients both treated and not treated with antihypertensive therapy, has been shown to be associated with increasing frailty as described by Ravindrarajah et al. in a population-based cohort study35. In addition, the FRAIL scale incorporates a number of co-morbidities that the FI does not account for including chronic lung disease and asthma, but also chronic kidney disease. In particular, both chronic lung disease and chronic kidney disease are associated with increased mortality36,37 and this may also explain why the FRAIL scale is more specific for the prediction of mortality.
Physiological dysregulation of the cardiovascular system in combination with frailty has been demonstrated to be independently associated with mortality in other longitudinal studies38. The frail treated BT for both FI and FRAIL had an increased risk of fall/fractures by Wave 2. As discussed earlier in relation to heart failure, both FI and FRAIL incorporate multiple cardiovascular co-morbidities, which combined with the physiological dysregulation of organ systems that occurs in frailty, can result in an increased risk of impaired BP regulation – in particular OH when the BP is already being treated to a low/intense level. OH at 40 seconds after standing and sustained OH in TILDA have been shown to be independently associated with recurrent, injurious, and unexplained falls39. On the other hand, the frail by FI with BP treated AT also had an increased risk of falls/fractures. This possibly reflects the fact that OH and low BP related events are not the sole mechanism of falls/fractures in frail older adults. The FI incorporates mobility assessment in its self-reported deficit questionnaire similar to the FRAIL scale. However, unlike the FRAIL scale, the FI also assesses transfers such as difficulty rising from a chair or difficulty “stooping, kneeling or crouching’’ and vision deficits which are all risk factors for falls40,41. This may explain the mechanism by which the frail by FI with BP treated AT were also at increased risk of falls/fractures. It should be noted that in our previous study, CFS and FP did not specifically incorporate cardiovascular co-morbidities, which may explain why they did not capture falls/fracture risk in the frail treated BT compared to the FRAIL/FI.
Our study has a number of limitations. Firstly, there is potential for misclassification of participants blood pressure control status at Wave 1 as BP readings used were solely based on the average of 2 readings and may not take into account variables such as white coat hypertension, BP variability etc., Secondly, the number of participants at baseline in Wave 1 that did not have complete data (638 in total) likely resulted in reduced statistical power when analysing the sub-groups we formulated. This reflects in some of the wide confidence intervals in some subgroup outcomes in the forest plots in Figure 3(a) and Figure 3(b). Future studies should include sub-group analysis for those with co-morbidities requiring specifically lower or higher BP treatment targets e.g. patient with diabetes, chronic kidney disease etc,. In addition, our data does not account for the burden of anti-hypertensive medication or doses of these respective medications. This nuanced analysis is not possible in our observational epidemiological design. Unfortunately, the reduced statistical power of our study did not allow us to perform these subgroup analyses’ of participant co-morbidities/medications and this is another limitation of our study. However, even though the number of frail participants according to FRAIL was the lowest (n=70), the FRAIL scale with BP treated BT had more statistically significant outcomes than any of the other frail/BP groups in this study. A further limitation is that TILDA is a study of community-dwelling adults and thus excludes those living in residential or nursing care. Frailty is highly prevalent in nursing home residents with rates varying depending on the frailty identification tools used: one study using the FI reported a prevalence of 81.6% for new male nursing home admissions42, while studies using the FP and CHSA-CFS reported proportions of 68.8% and 75.6% respectively43,44. Therefore, our study did not capture outcomes in the most frail. In addition, TILDA is based on an observational epidemiological longitudinal study design. Therefore, any significant results in this study are associations (not causation) that could potentially serve as the basis for future studies with higher power such as RCTs. In order to compare observational studies to RCTs on this topic, potentially a target trial design would need to be employed as observational studies are prone to biases45.
The current ESC/ESH guidelines do not specify what frailty tool to clinicians should utilise when assessing patients with potential frailty who have hypertension, and this is a significant limitation of these guidelines. A recent consensus statement published by Richter et al. on the topic of frailty in cardiology is a welcome development where the authors acknowledged the importance of explicit frailty screening in cardiology46. It is important to note that no specific frailty identification tool was recommended in their consensus document. Similarly, the ESC has recently acknowledged in The Cardiovascular Round Table forum the complexity of managing cardiovascular diseases in heterogeneous groups such as older adults. In particular, Lettino et al. have noted for frail older adults and those with functional dependence that anti-hypertensive treatment should be reassessed, and de-prescribing considered to avoid further deterioration in functional status and medication side effects47. Again, no specific frailty tool was recommended. Nevertheless, there is certainly an important conversation developing in the cardiology community and an acknowledgement that a patient-centred approach is needed for frail patients with cardiovascular diseases is welcome.
In this and our companion paper8, we compared four frailty classifications in their ability to predict 2-year incident adverse outcomes associated with below-target BP control (<130/70 mmHg) in The Irish Longitudinal Study on Ageing (TILDA). For the frail treated below target, hospitalisation by W2 was significantly more likely in those who were frail by FP, FI and FRAIL but not by CFS. The frail by FRAIL and BP treated below target were the only group with increased risk of mortality by W2. The frail by FI and FRAIL with BP treated below target had increased risk of hospitalisation, new heart failure and falls/fractures by W2.
Frailty was independently associated with adverse outcomes in hypertensive older adults treated below the ESC/ESH target. However, different frailty classifications had different prognostic implications. For those below the BP target, frailty by FRAIL was associated with the highest number of risks (falls/fractures, heart failure, hospitalisation and mortality), followed by the frail by FI (falls/fractures, heart failure and hospitalisation). Based on our results and frailty measures considered, the FRAIL and FI may be a superior frailty identification tool to utilise to identify frailty when applying the ESC/ESH guideline. Models of frailty that do not explicitly measure comorbidities (such as FP and CFS) may be less useful to capture risk of adverse events from lower blood pressure control.
Given the heterogeneity of the older, frail population we conclude that patients should be considered for comprehensive geriatric assessment once frailty has been identified (with any tool) to help guide their BP treatment. There needs to be larger, higher powered studies in clinical populations to definitively guide clinicians in this complex clinical scenario. Frailty has not yet proven in the few existing RCTs to be the ideal construct (versus other geriatric dimension such as cognition) for differentiating who might not benefit from more strict blood pressure control. These future studies should evaluate what clinical dimension will be best used to help differentiate those who may benefit versus from tight BP control versus those who won’t benefit.
The database from which the results were calculated and obtained cannot be shared due to data protection and ethical issues. Any requests to access the database can be made directly to TILDA (tilda@tcd.ie) and are considered on a case by case basis. The first two waves of TILDA data (upon which this papers analysis is based on) are available from the Irish Social Science Data Archive (ISSDA) at www.ucd.ie/issda/data/tilda/. To access the TILDA survey data, please complete an ISSDA Data Request Form for Research Purposes, sign it and send to ISSDA by email (issda@ucd.ie).
Figshare: Appendix - logistic regression analysis basic and full model for FRAIL and FI BP groups.docx. https://doi.org/10.6084/m9.figshare.1964307016.
This project contains the following extended data:
Appendix - logistic regression analysis basic and full model for FRAIL and FI BP groups.docx (This file contains the results tables of the basic and full binary logistic regression statistical analysis calculated for each Wave 1 frailty/blood pressure group (for Frailty index and FRAIL scale) and their respective risk for the seven Wave 2 health outcomes studied. These results are summarised in the main manuscript of the paper in Figure 2, Figure 3(a) and Figure 3(b))
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
This article is based on methodology first reported by Patrick O’Donoghue, Aisling M. O’Halloran, Rose Anne Kenny, Roman Romero-Ortuno, Do The Frail Experience More Adverse Events From Intensive Blood Pressure Control? A 2-Year Prospective Study In The Irish Longitudinal Study On Ageing (TILDA), Lancet e-Clinical Medicine, 2022, https://doi.org/10.1016/j.eclinm.2022.101304. The current article reports results with Frailty Index and FRAIL scale, while the previously published paper reported results based on Frailty Phenotype and Clinical Frailty Scale.
References
1. Masoli J, Delgado J, Pilling L, Strain D, et al.: Blood pressure in frail older adults: associations with cardiovascular outcomes and all-cause mortality. Age and Ageing. 2020; 49 (5): 807-813 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Blood pressure and cardiovascular ageing.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biostatistics, Frailty, Hypertension
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Blood pressure and cardiovascular ageing.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Madenci A, Wanis K, Cooper Z, Haneuse S, et al.: Strengthening Health Services Research Using Target Trial Emulation: An Application to Volume-Outcomes Studies. American Journal of Epidemiology. 2021; 190 (11): 2453-2460 Publisher Full TextCompeting Interests: I was an investigator involved in the Systolic Blood Pressure Intervention Trial (SPRINT).
Reviewer Expertise: Biostatistics, Frailty, Hypertension
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 25 Aug 22 |
read | read |
|
Version 1 09 Jun 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with HRB Open Research
Already registered? Sign in
Submission to HRB Open Research is open to all HRB grantholders or people working on a HRB-funded/co-funded grant on or since 1 January 2017. Sign up for information about developments, publishing and publications from HRB Open Research.
We'll keep you updated on any major new updates to HRB Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)