Keywords
Attention, Cognitive rehabilitation, Executive function, Intervention studies, Memory, Outcome and process assessment, Pilot RCT, Post-stroke cognitive impairment, Protocol, Psychoeducation, Stroke
Post-stroke cognitive impairment (PSCI) affects approximately 40% of stroke survivors within one year, yet cognitive rehabilitation is not routinely integrated into post-stroke care. This protocol outlines a pilot randomised controlled trial (RCT) to assess the feasibility, acceptability, and preliminary effectiveness of a structured cognitive rehabilitation programme, StrokeCog-R, for PSCI. A nested Study Within A Trial (SWAT) will evaluate the impact of follow-up communication methods on participant retention.
Sixty-four stroke survivors will be recruited and randomly assigned to either a StrokeCog-R intervention (n= 32) or control (usual care; n= 32) group. StrokeCog-R is a five-week, group-based cognitive rehabilitation/psychoeducation programme targeting executive function, memory, and attention, delivered by a clinical neuropsychologist. Primary outcomes include recruitment, retention, intervention acceptability, and cognition. Cognitive function will be assessed using the National Institute for Neurological Disorders and Stroke (NINDS) battery at baseline (six weeks post-stroke), immediately post-intervention/control period, and at a four-month follow-up. Secondary outcomes include self-efficacy, functional ability, mood, as well as carer-rated cognitive and behavioural changes and carer wellbeing. The SWAT will examine whether additional text messages during the follow-up period improve retention at the four-month assessment compared to telephone calls alone. Recruitment and retention rates will be reported with 95% confidence intervals. Effect size estimates from pre- to post-intervention changes in cognition will inform sample size calculations for a future definitive trial. Qualitative data will be analysed using a reflexive thematic approach, guided by the Consolidated Framework for Implementation Research (CFIR).
This pilot RCT will assess the feasibility of conducting a full-scale definitive RCT, including recruitment and retention rates, and preliminary outcome trends. SWAT findings will guide future retention strategies.
Findings will inform the design of a larger RCT and, if feasible, support the potential integration of cognitive rehabilitation into routine post-stroke care to improve long-term cognitive outcomes.
Attention, Cognitive rehabilitation, Executive function, Intervention studies, Memory, Outcome and process assessment, Pilot RCT, Post-stroke cognitive impairment, Protocol, Psychoeducation, Stroke
Stroke is the second leading cause of death globally, following ischaemic heart disease, and remains a major contributor to acquired disability1,2. As global populations age, stroke has emerged as a critical public health challenge3. Although age-standardised stroke mortality rates have declined over the past two decades, the absolute number of stroke cases, survivors, and the overall global burden of stroke continue to rise1,4. This increasing prevalence places substantial economic strain on healthcare systems and heightens demands for medical care, community support, and long-term rehabilitation services5,6.
Stroke can lead to a wide range of physical, emotional, and cognitive impairments that significantly impact a survivor’s quality of life. Indeed, post-stroke cognitive impairment (PSCI) is common, manifesting as either focal deficits (e.g., naming or hemi-spatial neglect) or global impairments affecting memory, attention, learning, decision-making, and problem-solving7,8. Cognitive impairment is defined as self- or informant-reported cognitive complaints, objective deficits, preserved functional independence, and the absence of a dementia syndrome9. While most cases are mild-to-moderate in severity10, severe cognitive impairment can develop, particularly after recurrent strokes11,12. Notably, over 50% of stroke survivors experience PSCI at six months post-stroke10, with 38% still affected at one year despite not meeting dementia criteria13. However, these estimates likely underrepresent the true burden due to practical challenges such as feasibility of testing and follow-up attrition11.
Beyond cognitive decline, stroke survivors frequently experience emotional and psychological challenges14,15, which can hinder their reintegration into social and occupational roles. These impairments often limit participation in meaningful daily activities16,17 and contribute to increased disability, reduced independence10, and long-term morbidity and mortality18. Additionally, cognitive impairment can interfere with adherence to medication and rehabilitation regimens18, emphasising the need for targeted cognitive interventions. Beyond its direct impact on stroke survivors, PSCI places substantial strain on caregivers, increasing disability and reducing overall quality of life19. There is also a strong correlation between cognitive impairment and the need for long-term care, particularly among individuals with severe post-stroke disabilities6.
Stroke and dementia exhibit a bidirectional relationship; experiencing a stroke elevates the risk of cognitive decline, while pre-existing dementia exacerbates the likelihood of stroke-related complications12. Optimal acute stroke management and secondary prevention are therefore crucial in mitigating cognitive deficits and reducing the risk of recurrent stroke12. A systematic review and meta-analysis found that 10% of patients had dementia before their first stroke, another 10% developed dementia within one-year post-stroke, and more than one-third developed dementia after recurrent stroke12. More recent analyses estimate that approximately one in five stroke survivors develop dementia (type, unspecified), with the highest risk occurring within the first year, particularly following recurrent strokes11. The Oxford Vascular Study further elucidates this risk, demonstrating that the incidence of dementia within one year post-event was 34% for patients with severe stroke, 8% for those with minor stroke, and 5% following transient ischaemic attack20. These findings indicate a significant increase in dementia risk even after less severe cerebrovascular events, compared to the general population, underscoring the need for interventions across all severities of stroke20.
Variation in reported prevalence is largely due to methodological differences across studies, including whether pre-stroke dementia is accounted for, differing diagnostic criteria, and the heterogeneity of stroke and dementia subtypes, many of which are not clearly specified11,12,20. Indeed, the term “poststroke dementia” is commonly applied to any dementia developing after a clinically evident stroke, without necessarily indicating a specific underlying neuropathological mechanism21. This practical convention acknowledges that cognitive impairment post-stroke often reflects a blend of vascular damage and neurodegenerative processes22.
Building upon this understanding, the convergence of an ageing population and increasing post-stroke survival rates suggests that the prevalence of post-stroke dementia is expected to rise, as both stroke and dementia risks escalate with advancing age23,24. These findings underscore the critical need for effective secondary prevention strategies to reduce the burden of PSCI and dementia. As research increasingly focuses on modifiable risk factors for dementia, PSCI has emerged as a crucial target for preventive strategies25. Indeed, addressing PSCI has been identified as a key priority for stroke survivors, caregivers, and healthcare professionals26–28.
While substantial research has been dedicated to physical rehabilitation post-stroke, leading to significant functional improvements29, cognitive rehabilitation remains relatively underexplored by contrast, with evidence for its efficacy not well established30. Cognitive rehabilitation involves systematic, functionally orientated therapeutic activities guided by cognitive assessment and an understanding of the relationship between brain injury and behaviour31. It addresses multiple cognitive domains, including attention, memory, perception, and executive functioning (e.g., cognitive flexibility, problem-solving, planning, and self-regulation). Despite its potential benefits, five Cochrane reviews examining post-stroke cognitive rehabilitation across these domains, focusing on occupational therapy for cognitive impairment8 and interventions targeting executive dysfunction32, attention deficits33, memory deficits34, and spatial neglect35, concluded that evidence for its efficacy remains insufficient. Consequently, the effectiveness of cognitive rehabilitation interventions targeting isolated cognitive domains remains uncertain.
PSCI often manifests as deficits across multiple cognitive domains rather than being confined to a single function36,37. The severity of cognitive impairment is further influenced by lesion location, which can affect multiple cognitive domains38,39. A systematic review of 22 trials, including nine focusing on non-core domains such as perception and language, found a small effect of cognitive rehabilitation on general cognitive impairment40. Similarly, a review of non-randomised controlled trials assessing psychological interventions for PSCI reported a small effect on global cognition30, though these studies exhibited low quality and high risk of bias. These findings highlight a significant lack of high-quality randomised controlled trials (RCTs) in this area, underscoring the need for further rigorous research to strengthen the evidence base.
In Ireland, the lack of cognitive rehabilitation and psychological support in standard stroke care has been flagged as a key issue in the National Stroke Strategy 2022–20272. The National Institute for Health and Care Excellence (NICE) guidelines recommend that all stroke patients be screened for cognitive impairment within two weeks of stroke onset, irrespective of severity, to identify initial cognitive deficits41. If impairment is detected, a full cognitive assessment should be conducted six weeks post-stroke41–43. However, evidence suggests that cognitive assessments are not routinely conducted in Ireland or internationally. The 2022 National Stroke Audit44 highlights a severe shortage of psychological services for patients with PSCI, a problem unchanged since the first audit in 2006–200745. While 93% of patients are assessed by physiotherapists, only 7% are assessed by psychologists44, and just 21% of hospitals provide access to clinical psychologists as part of routine stroke unit care46. The absence of structured cognitive rehabilitation programmes, coupled with the significant shortage of psychological services, highlights a crucial gap in post-stroke care, emphasising the urgent need for targeted interventions.
In response to this critical gap in post-stroke cognitive rehabilitation, the original StrokeCog study aimed to model and mitigate the impact of PSCI through targeted intervention, with a key objective of developing and evaluating an effective cognitive rehabilitation programme47. The StrokeCog-R intervention was created using the Medical Research Council (MRC) framework for complex interventions48,49. Given the multifaceted nature of cognitive rehabilitation (shaped by factors such as fatigue, emotional adjustment, impairment awareness, and functional capacity), a structured, evidence-based approach was essential. The intervention’s design was informed by a systematic review, qualitative interviews with 44 stakeholders (including stroke survivors, carers, and healthcare professionals), and feasibility testing through n-of-1 studies to evaluate its real-world applicability28,30,50,51.
Findings from this development phase highlighted the need for structured yet flexible interventions that addressed multiple cognitive domains while accommodating individual needs28,30,50. Stroke survivors reported that cognitive deficits significantly impacted daily life, confidence, and self-efficacy, emphasising the importance of psychoeducation, fatigue management, adjustment strategies, and goal-oriented rehabilitation. Regaining confidence in cognitive and functional abilities emerged as a central theme28, with research linking confidence to self-efficacy52, a top priority for stroke survivors, caregivers, and healthcare professionals26,27. As self-efficacy is a key predictor of rehabilitation success53, it was made central to the StrokeCog-R intervention. Systematic reviews further reinforce self-efficacy’s role in post-stroke adjustment54, while goal-setting and action planning empower patients to manage cognitive challenges55,56. Participants also expressed a strong preference for interventions tailored to cognitive severity, fatigue, mood fluctuations, and personal goals. To address this, StrokeCog-R integrates both group-based and individualised components, balancing peer support with personalised rehabilitation strategies to enhance self-efficacy, psychological adjustment, and long-term cognitive recovery. Another key recommendation from the development phase was that cognitive rehabilitation should be delivered by healthcare professionals trained in both cognitive rehabilitation and post-stroke adjustment. Clinical neuropsychologists, given their expertise, were identified as ideal facilitators. However, they remain underrepresented in stroke rehabilitation teams both nationally and internationally44,45.
The resulting StrokeCog-R intervention consists of a five-week group-based programme led by a clinical neuropsychologist50. Sessions, lasting 2.5 hours with a mid-session break, accommodate four patients and combine cognitive training with psychoeducation. Home-based activities are personalised to facilitate skill transfer into daily life. In addition to cognitive rehabilitation, the intervention prioritises psychological adjustment through stress management, fatigue management, and self-efficacy strategies. The intervention draws from evidence-based rehabilitation approaches in traumatic brain injury rehabilitation57–62 and incorporates expertise from clinicians specialising in neuropsychology and secondary stroke prevention. The intervention is grounded in a biopsychosocial model63, recognising the interplay between cognitive impairment, daily activities, and social participation. The WHO International Classification of Functioning, Disability, and Health (ICF)64 further informs its approach, emphasising the need to assess not just impairment but also its impact on meaningful activities and participation65. A thorough clinical and cognitive assessment ensures that rehabilitation aligns with the individual’s premorbid function, current needs, and personal goals66.
Feasibility testing employed an n-of-1 study design to assess the intervention’s acceptability and practicality50,51. Two groups participated: one with mild-to-moderate PSCI and another with severe PSCI alongside their caregivers. Each participant engaged in group-based rehabilitation supplemented by individualised home exercises. The study confirmed the intervention's suitability for individuals with mild-to-moderate PSCI, with participants reporting subjective improvements in cognitive function and self-efficacy. They found the intervention’s content, duration, and dose acceptable. However, while caregivers of stroke survivors with severe PSCI found the content informative, the survivors themselves reported being unable to engage with the intervention, even at a reduced duration. Further, recruitment challenges emerged as a key barrier, as clinical staff with existing workloads managed patient recruitment, leading to delays and participant withdrawals before intervention commencement. However, once initiated, retention was high. These findings informed refinements for the present pilot RCT methodology. To enhance recruitment efficiency, a dedicated research assistant will oversee patient enrolment, reducing the burden on clinical staff. The need for protected time for clinical neuropsychologists to deliver the intervention was also identified. Moreover, inclusion criteria were refined to focus on stroke survivors with mild-to-moderate PSCI.
Pilot RCT: StrokeCog-R. This protocol outlines the pilot RCT which aims to assess the feasibility and acceptability of delivering the StrokeCog-R cognitive rehabilitation intervention for stroke survivors with mild-to-moderate cognitive impairment. Specifically, the primary focus is to evaluate the implementation of StrokeCog-R by examining recruitment, retention, follow-up processes, and cognitive outcomes. Additionally, the study will estimate the effect size (based on between-group changes in cognition over time) to inform the design of a future definitive trial comparing StrokeCog-R to usual post-stroke care in Ireland. This study aligns with Step 3 of the Medical Research Council (MRC) Framework for Developing and Evaluating Complex Interventions48,49. A qualitative process evaluation will explore the acceptability of the intervention to patients and patient adherence to the study protocol, with findings used to refine the protocol for a larger trial.
Secondary objectives include exploratory analyses of self-efficacy outcomes. Additionally, a micro-costing analysis will examine material and consumable expenses, as well as staffing costs for recruitment, assessments, and intervention delivery. Healthcare utilisation costs recorded for both the intervention and control groups will also provide insights into the cost of usual care and intervention participation for the purpose of developing and costing a larger definitive trial.
Study Within a Trial (SWAT): Retention Strategies. As part of the pilot RCT, we will conduct a Study Within a Trial (SWAT) to evaluate the effectiveness of communication strategies in improving participant retention at follow-up. Retention remains a significant challenge in clinical trials, with a median loss-to-follow-up of 11% reported in UK trials67, and it is a top priority for methodological research68. The SWAT will investigate the effectiveness of different communication strategies in improving participant retention at follow-up. The primary research question is whether supplementing follow-up telephone calls with summary text messages enhances retention compared to telephone calls alone. Given the cognitive challenges faced by stroke survivors, written summaries may improve recall and engagement, ultimately reducing loss to follow-up. This study aligns with the PRioRiTy II research agenda, specifically addressing the question of how technology can optimise trial follow-up processes69. Findings will contribute to trial methodology by informing best practices for participant retention in post-stroke cognitive rehabilitation research and will support the design of a future definitive StrokeCog-R trial.
This pilot RCT will evaluate a 5-week, group-based cognitive rehabilitation intervention. Sixty-four stroke survivors with mild-to-moderate cognitive impairment will be randomised to either a StrokeCog-R intervention or control group. The pilot RCT was registered at clinicaltrials.gov (NCT06021470) and ethical approval was granted by the Beaumont Hospital Research Ethics Committee (REC reference: 23/14), which has oversight over both recruitment settings (Beaumont Hospital and St. Joseph’s Rehabilitation Hospital, Dublin, Ireland). Additionally, the SWAT will be conducted during the follow-up period of the pilot RCT. Participants within both intervention and control groups will undergo further randomisation to the SWAT intervention, allowing for additional evaluation of specific trial processes. The SWAT is registered in the SWAT repository at Queen’s University Belfast (SWAT232;70). We used the SPIRIT checklist when writing this report71.
Recruitment will be conducted at Beaumont Hospital and St. Joseph’s Rehabilitation Hospital in Dublin, Ireland, while assessments and interventions will take place at the Clinical Research Centre in Beaumont Hospital.
This pilot RCT aims to recruit 64 stroke survivors diagnosed with mild-to-moderate PSCI, with equal allocation to the intervention and control groups. While the study is not powered to detect a significant effect on the primary outcome (cognitive function, specified below), it will provide an estimate of the standard deviation (SD) necessary for calculating effect size in a future definitive trial. Additionally, whenever possible, a caregiver or family member will be invited to participate by completing questionnaires, with one respondent per participant (64 caregivers/family members). Additionally, the staff members involved in delivering the intervention will participate in a qualitative process evaluation following intervention delivery.
Sim et al.72 recommend a minimum of 50 participants to estimate SD with sufficient precision; with 64 participants, this study meets that threshold, ensuring reliable data for informing a larger trial. Additionally, a minimum sample of 60 participants is required to estimate a recruitment or retention rate of at least 50% with a precision of 12.7%. Recruitment feasibility is supported by stroke admission rates, with 433 admissions to the Beaumont Hospital stroke unit in 202273. Considering a 15–20% mortality rate and that 38% of surviving patients present with cognitive impairment13, a recruitment rate of six participants per month is expected. Based on prior research and national audit data, this enrolment rate would enable completion of study recruitment within 12 months. To support participant adherence, recruitment and retention success is defined as retaining at least 90% of those randomised at the 4-month follow-up assessment.
To ensure a rigorous selection process, recruitment will follow a structured, multi-stage eligibility procedure (see Figure 1).
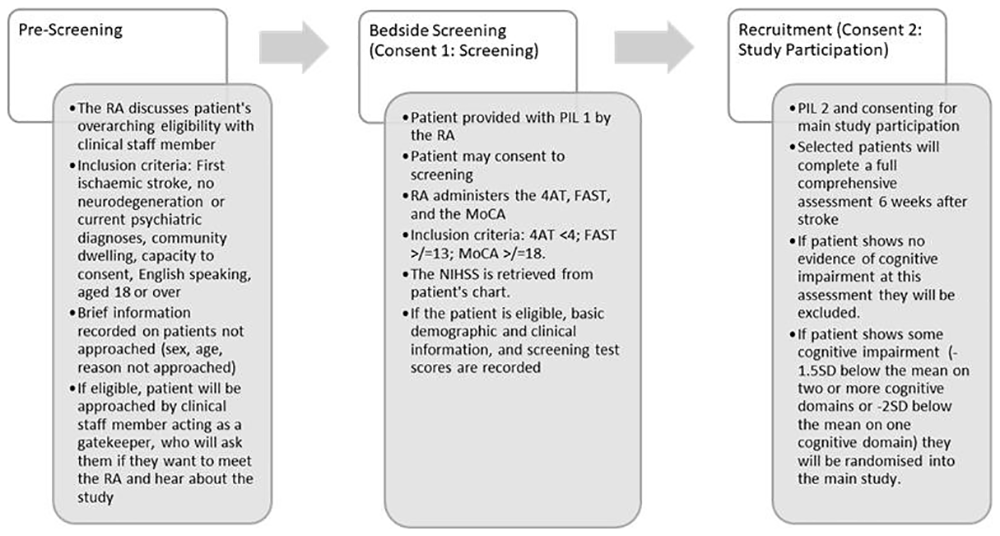
Note. Abbreviations: 4AT, Assessment Test for Delirium and Cognitive Impairment FAST; Frenchay Aphasia Screening Test; MoCA, Montreal Cognitive Assessment; NIHSS: National Institutes of Health Stroke Scale; PIL, participant information leaflet; RA, research assistant; SD, standard deviation.
Stage 1: Pre-screening. A research assistant (RA) will recruit consecutive stroke patients from both an acute hospital setting (Beaumont Hospital) and a post-acute rehabilitation facility (St. Joseph’s Hospital). Patient identification will be facilitated through collaboration with a multidisciplinary stroke team. A pre-screening assessment, conducted in consultation with clinical staff, will establish overarching eligibility, ensuring that patients who do not meet inclusion criteria are not approached. Initial inclusion criteria include prospective participants who have experienced a first-ever ischaemic stroke, are aged 18 or older, reside in the community rather than in long-term care facilities, speak and understand English, and have the capacity to provide informed consent. Additionally, participants must have no history of neurodegenerative disorders or psychiatric conditions as defined by DSM-5 or ICD-11 criteria. Eligible patients will be introduced to the study by a clinical team member acting as a gatekeeper. The RA will then provide further details, including a participant information leaflet (PIL).
Stage 2: Bedside screening. Patients expressing interest in participation will provide written informed consent for screening. Adequate time will be given to review the PIL, ask questions, and sign the first consent form, which will apply solely to the screening process. Patients deemed eligible post-screening will undergo an additional consenting process before formal enrolment in the main trial. Participants will first be screened for delirium using the 4AT (Assessment Test for Delirium and Cognitive Impairment)74, with a score below 4 indicating eligibility. If delirium is detected, screening will be halted. Next, participants will be screened for aphasia using the Frenchay Aphasia Screening Test (FAST)75, with a minimum score of 13 required for eligibility. If no significant aphasia or delirium is present, participants will then complete the Montreal Cognitive Assessment (MoCA)76, with a minimum score of 18 required for inclusion (permission for its use was obtained 20th September 2023, https://mocacognition.com). A score of 18 or above indicates either mild cognitive impairment (MCI) or normal cognitive performance76. Those meeting this criterion will be initially included, with the understanding that a comprehensive neuropsychological assessment will be conducted six weeks post-stroke. This will help identify more subtle cognitive deficits that may not be apparent during the initial screening or account for cognitive fluctuations in the early period following a stroke77. Patients failing to meet eligibility criteria due to more severe cognitive impairment, significant aphasia, or current delirium will be excluded from the study. These patients will be informed of the outcome and thanked for their participation. Those patients meeting all screening requirements will be invited to proceed with participation.
Baseline data will be collected for all screened participants, including demographic information such as age, sex, highest level of education, and employment status. Clinical details will also be extracted from the patient’s medical records, including stroke history, early intervention information, multimorbidity, clinical frailty, and stroke severity as measured by the National Institutes of Health Stroke Scale (NIHSS;78). All screening scores and patient data will be recorded using a pseudonymous alphanumeric code to maintain confidentiality. For individuals found to be ineligible following screening, the reason for exclusion will be documented.
Stage 3: Baseline neuropsychological assessment. Participants will undergo a full neuropsychological evaluation six weeks post-stroke, in line with NICE recommendations, which recommend that cognitive screening be followed by a detailed assessment using valid and reliable tools before initiating a treatment plan41–43. For participants discharged from the hospital, assessments will take place at a Clinical Research Centre, with transport arranged by the study team. Home-based assessments will be offered for individuals with mobility challenges. Patients screened in the rehabilitation setting will undergo full neuropsychological assessments within one week of their bedside screening if they are six weeks or more post-stroke.
Before proceeding with the full baseline assessment, participants will complete a second informed consent process specific to their enrolment in the main pilot RCT. They will then undergo a comprehensive 60-minute neuropsychological battery based on the National Institute of Neurological Disorders and Stroke (NINDS) cognitive assessment battery79 and some additional measures (see §Outcome Measures). This assessment will evaluate core cognitive domains, including memory, attention, executive function, and information processing speed. Norm-referenced test scores, using norms from the test publishers, will determine final eligibility. Participants will be included if they score at least 1.5SD below the mean in two or more cognitive domains or at least 2SD below the mean in one cognitive domain, consistent with established criteria used in other MCI and post-stroke cognitive studies80,81. Those with no detectable cognitive impairment following this assessment will be excluded from further study participation and will be thanked for their time. Participants meeting the cognitive impairment criteria will proceed to randomisation.
For each eligible patient participant, a family member or caregiver will be invited to take part in the study. The patient will be asked if they have a family member or carer who is interested in participating. If the individual expresses interest in participating, they will receive a PIL and provide written consent, which can be returned via a pre-stamped, addressed envelope. If the family member or caregiver is available in person, they will sign the consent form on-site. Standardised questionnaire measures will then be sent by post, and can either be returned using a separate pre-stamped, addressed envelope or brought to the participant's subsequent testing session.
Finally, staff members involved in delivering the intervention will be invited to participate in a qualitative process evaluation. They will be provided with a PIL and will give written informed consent if they choose to take part.
Following the baseline assessment, eligible participants will be randomly assigned to either the intervention or usual care group by the RA. A total of 64 stroke survivors will be randomised, with equal allocation to the intervention (n= 32) and control (n= 32) groups using a computer-generated randomisation sequence. Randomisation will be conducted by an independent statistician (KB) to ensure allocation concealment. The randomisation sequence will be generated using the envelope randomisation82 to create a block randomisation list for the 1:1 randomisation to intervention or control group using block sizes 4, 6, and 8. In addition, within the intervention and control arms, a further randomisation sequence will be generated for the allocation to the SWAT group in a 1:1 ratio, using blocked randomisation with block sizes 4, 6 and 8. To ensure the concealment of the allocation, the randomisation sequence will only be available to the researcher who is involved in recruiting the participants into the study. The researcher conducting all post- and follow-up neuropsychological assessments will remain blinded and will not be involved in the intervention delivery. Since participants cannot be blinded to their group assignment, objective cognitive measures will be used to minimise response bias. To further preserve blinding, participants will be instructed not to disclose their allocation during post-assessments. The clinical neuropsychologist delivering the intervention will have no role in assessments. All participant data will be pseudonymised initially using unique alphanumerical identifiers for pre-, post-, and follow-up assessment analyses, until eventual irrevocable anonymisation following study completion.
StrokeCog-R intervention group. The intervention will be delivered in small groups of four participants at Beaumont Hospital, Dublin, Ireland by a clinical neuropsychologist. The 5-week programme combines psychoeducation with compensatory cognitive strategies to target executive function, memory, and attention. Each session lasts approximately 2.5 hours, including a break of 30 minutes with refreshments, and incorporates both rehearsal-based and compensatory-based strategies to enhance cognitive functioning. Sessions include brief presentations on stroke-related cognitive deficits, along with discussion and practical strategies for managing these challenges. Between sessions, participants engage in tailored home activities designed to reinforce skill application, promote self-efficacy, and support the achievement of personally identified goals. To maintain engagement, a research team member conducts weekly follow-up phone calls to assess participation, self-efficacy, and general well-being, as well as providing additional support for home activities where necessary. The content of each session is outlined below.
Session 1 – Introduction and Psychoeducation: This first session welcomes participants and provides an overview of the StrokeCog-R cognitive rehabilitation programme. Key topics include the causes and impact of different stroke types, secondary prevention and lifestyle modifications, post-stroke adjustment, common mood and behavioural changes including fatigue, and available support resources. The session also introduces action planning, emotional well-being strategies, and mindfulness. Additionally, participants complete a self-report questionnaire on executive functioning in preparation for the next session.
Session 2 – Executive Function: This session begins with a review of the previous week’s material and home-based activities. Participants learn about the brain regions involved in executive function and common difficulties with planning and organisation following stroke. Various compensatory strategies are introduced, including environmental modifications, internal cognitive techniques, and external memory aids. Participants share real-life experiences, engage in problem-solving activities, and identify two to three strategies to implement at home. A self-report questionnaire on memory is completed in preparation for the next session.
Session 3 – Memory: Following a review of the previous session, this session focuses on different types of memory and the brain areas involved in encoding, storing, and retrieving information. Participants learn about common memory deficits after stroke and explore strategies for improving recall, including environmental restructuring and the use of internal and external memory aids. Practical exercises and problem-solving discussions reinforce these strategies. Participants select two to three strategies to practice at home and complete a self-report questionnaire on attention for the next session.
Session 4 – Attention: This session covers the different types of attention, the brain regions responsible for attention control, and common attention and concentration difficulties following stroke. Participants are introduced to strategies for managing attentional deficits, including environmental adjustments and cognitive compensatory techniques. Real-life experiences are discussed, and problem-solving exercises are used to help participants integrate these strategies into their daily lives. Participants develop an action plan for continued practice at home.
Session 5 – Emotional Well-Being and Consolidation of Learning: The final session revisits stroke-related psychoeducation from Session 1 and reviews participants’ experiences with the strategies introduced throughout the programme. The discussion focuses on the psychological impact of stroke, common mood changes, managing fatigue, and strategies for emotional regulation and well-being. Participants develop a long-term plan to maintain and apply the cognitive strategies they have learned and discuss approaches to managing future stroke-related challenges.
Control group: usual care. Participants in the control group will receive their usual standard stroke rehabilitation care. The nature and intensity of usual care may vary between acute hospital and rehabilitation settings. To account for these variations and also to inform a micro-costing analysis, detailed information on healthcare utilisation will be recorded at each assessment session for both the intervention and control groups.
To maintain engagement, a member of the research team also makes weekly follow-up phone calls to the control group during the intervention/control period, coinciding with the timing of calls to those in the intervention group, to assess participation and check on general well-being. Upon study completion (i.e., once all intervention groups have concluded and control group participants have completed their follow-up assessments), individuals in the control group will receive a video-recorded version of the StrokeCog-R intervention along with all accompanying materials.
SWAT intervention group. During the four-month period between the post-intervention and follow-up assessments, participants in the SWAT intervention group will receive two follow-up telephone calls, each accompanied by a summary text message. The first call will take place one month after the immediate post-intervention assessment, while the second will occur one month prior to the final four-month follow-up assessment. These calls will follow a structured script that includes: (a) an expression of appreciation for their participation in the StrokeCog-R trial; (b) a reminder of the importance of continued involvement; (c) details of the next scheduled contact before the follow-up assessment; (d) information on the expected timeline for trial results and how they will be communicated; and (e) a reminder that participants can contact the StrokeCog team with any questions or concerns. Following each call, participants will receive a summary text message reiterating these key points.
SWAT control group. Participants in the SWAT control group will also receive two follow-up telephone calls at the same intervals as those in the intervention group. These calls will follow an identical scripted format; however, they will not receive any supplementary summary text messages.
Patient assessment: primary and secondary outcome measures. Patients will undergo cognitive and behavioural evaluations at three time points: baseline (six weeks post-stroke, pre-intervention), immediately post-intervention (or equivalent control period), and at a 4-month follow-up to examine maintenance effects. The primary outcome is cognitive function and will be measured using a modified version of the validated 60-minute NINDS cognitive battery79. This comprehensive battery evaluates key cognitive domains, including memory, attention, executive function, and information processing. These assessments will include the Digit Span (Forward, Backward, and Sequencing trials), Verbal Fluency Test (Phonemic and Semantic), Colour Trail Tests (A & B), 30-Item Boston Naming Test, Symbol Digit Modalities Test, Rey Complex Figure Test (Copy, Immediate recall, Delayed Recall, and Recognition), and the Rey Auditory Verbal Learning Test (Immediate recall, Delayed Recall, and Recognition). To supplement these measures, participants will complete the Test of Premorbid Functioning (TOPF;83) at baseline only, while the Wechsler Abbreviated Scale of Intelligence Second Edition (WASI-II84) Vocabulary and Matrix Reasoning tests will be administered at every assessment. These assessments align with routine clinical practice in Beaumont Hospital’s Department of Psychology and are well tolerated by patients.
A key secondary outcome is patient-reported self-efficacy in managing cognitive deficits, assessed using the Modified Stroke Self-Efficacy Scale (MSSES;85). Additional measures will include demographic and clinical information. As per best practice21, cognitive testing will be complemented by functional assessments (WHO Disability Assessment Schedule, WHODAS-12;86), Modified Rankin Scale (MRS;87,88) and evaluations of frequently co-occurring neuropsychological issues, including mood (Generalized Anxiety Disorder 7-item assessment, GAD-789); Patient Health Questionnaire (PHQ-890), and fatigue (Fatigue Severity Scale, FSS91). Participants will also be asked to identify and prioritise their goals for subjective cognitive and psychological difficulties (Schedule for the Evaluation of Individual Quality of Life (SEIQoL)92.
Family Member or Carer Assessment. Family members or carers who are available and willing to participate will complete structured evaluations to provide insight into the patient’s cognitive and behavioural changes post-stroke. These assessments will include questionnaires on perceived cognitive decline and behavioural changes of the stroke survivor from the family member’s perspective (Informant Questionnaire for Cognitive Decline in the Elderly (Short Form IQ-CODE)93). Additionally, carers will complete validated assessments measuring their psychological well-being including the GAD-7, PHQ-8, Perceived Stress Scale (PSS;94), caregiver burden (the 22-item Zarit Burden Interview95), and vulnerability (Vulnerable Elders Survey (VES96). These data will help assess the broader impact of stroke-related cognitive impairments on both patients and their support systems.
Qualitative Process Evaluation. In line with MRC guidelines for evaluating complex interventions48,49, qualitative focus group interviews with participants in the intervention group will assess the acceptability and feasibility of the intervention, as well as adherence. These discussions will explore their experiences, perceived benefits, and any challenges associated with the intervention. These sessions will take place at the conclusion of the final intervention session, prior to the post-intervention assessment. Additionally, qualitative data will be collected from staff delivering the intervention to explore their perspectives on feasibility, usefulness, and any necessary refinements before scaling up to a definitive trial. To ensure protocol fidelity, the intervention will follow a manualised format delivered by a clinical neuropsychologist. Any deviations from the protocol, such as additional tailoring, will be documented. Process notes will be maintained to assess adherence and identify any areas for refinement.
Micro-Costing. A micro-costing exercise will be conducted throughout the trial to estimate the costs associated with scaling the intervention into a future definitive trial. This bottom-up approach involves systematically collecting data on resource utilisation, including the clinical neuropsychologist’s time, educational materials, and participant-related expenses such as transportation. Additionally, at each testing session, we will record all participants’ healthcare utilisation, informal care, and any social support expenses.
SWAT Outcome Measures. The SWAT primary outcomes include retention to the trial at the 4-month final post-intervention assessment, comparing participants who received additional text summaries with those who did not. Additionally, qualitative data, via self-report questionnaires, will be collected at the final assessment to assess the acceptability and perceived effectiveness of the additional text follow-up. The secondary outcomes are the cost per participant retained, and the proportion of participants withdrawing prior to the final assessment.
To address the primary research question regarding recruitment and retention, we will assess recruitment rates for all screened patients, presenting these rates by recruitment approach along with corresponding 95% confidence intervals (CIs). For participants who meet the inclusion criteria, we will calculate the proportions randomised and retained at each post-intervention assessment, also with 95% CIs. Patient characteristics will be analysed to identify factors associated with attrition. Descriptive analyses will be performed, and clinically meaningful changes in the primary outcome (cognitive function) from pre- to post-assessment will be indicated by standardised effect sizes ranging from 0.3 to 0.5, based on evidence from previous systematic reviews30,40. Changes in cognitive outcomes between groups over time will be used to estimate effect sizes. As this is a pilot study, statistical significance will be considered exploratory, with findings primarily informing the estimation of the standard deviation (SD) needed to power a future definitive trial. Additionally, the cost of delivering the intervention will be calculated, with 95% CIs reported. All quantitative analyses will be performed using Stata software.
To evaluate intervention acceptability, we will transcribe and analyse qualitative data from the process evaluation involving patient participants in the intervention group and staff members responsible for delivering the intervention. Reflexive thematic analysis97,98 will be employed using an inductive, reflective approach. The analysis will also be informed by the Consolidated Framework for Implementation Research (CFIR), which includes five domains: intervention characteristics, outer setting, inner setting, characteristics of individuals involved, and the implementation process99, as well as the MRC framework for complex interventions48,49. Thematic analysis will be conducted using NVivo software.
For the SWAT analysis, we will report the proportion of patients withdrawing before the 4-month post-intervention assessment as numbers and percentages. Statistical comparisons between the SWAT intervention and control groups will be made using chi-square analysis. Qualitative data from the questionnaire self-report process evaluation at the end of the final assessment will also contribute to the analysis. Secondary analysis will assess the cost per participant retained between the group receiving both telephone follow-up and text summary versus those receiving telephone calls alone. This will include an evaluation of costs associated with telephone calls, texts, and staff time spent administering the intervention.
For the purposes of data analysis related to adherence, recruitment and retention success is operationally defined as retaining at least 90% of randomised participants at the 4-month follow-up assessment. To evaluate strategies for improving adherence, the SWAT intervention70 will examine the impact of enhanced communication methods delivered between the post-intervention assessment and the 4-month follow-up on participant retention rates. Complementing the quantitative analysis, a qualitative process evaluation will explore patterns of adherence to the study protocol and identify perceived barriers to continued participation.
Sex and Gender Data Analysis. There are known sex differences in stroke incidence and outcomes, with the incidence rising in older women and greater risk for poorer post-stroke recovery100. Additionally, evidence from cardiac rehabilitation suggests lower uptake and poorer attendance rates in women, a trend that has not improved over time101,102. To account for potential sex and gender differences, all data analysis and reporting will disaggregate findings by sex and gender, where feasible. The trial will explore any potential disparities in participation, retention, reasons for attrition, and outcomes, ensuring the research addresses the needs and experiences of all participants.
Patient and Public Involvement (PPI) will play a central role in this study, with ongoing contributions from patients who have experienced stroke and PSCI, as well as their family members. Their involvement spans multiple stages of the trial, including the previous development and feasibility testing of the StrokeCog-R intervention, co-designing participant materials, offering input on the ethical approval process, interpreting results, and developing creative strategies for disseminating research findings50. A key member of the Trial Steering Committee, who has lived with PSCI, has contributed to the development of this study, ensuring the perspectives of those directly affected by stroke are integrated into every phase. PPI will also inform decisions such as tailoring the intervention to individual patient goals, reducing reliance on written information, and assessing the impact of telephone-based follow-up.
Ethical approval for the trial has been granted by the Beaumont Hospital Research Ethics Committee (REC reference: 23/14) and a data protection assessment has been approved by the Data Protection Officers at both RCSI University and Beaumont Hospital. Ethical considerations for this pilot trial have been carefully addressed to ensure participant protection and compliance with relevant laws and regulations. Participants will be assigned a pseudonymous alphanumeric ID to ensure confidentiality, and a master list linking participant names with IDs will be securely maintained and then deleted at the end of the study, whereupon data will be irrevocably anonymised. Data handling will comply with GDPR (Regulation (EU) 2016/679)103 and the Data Protection Act 2018 (Section 36(2)) (Health Research) Regulations 2018)104, with all electronic files encrypted and password-protected. Hard copies will be minimised; where necessary, documents will be scanned, stored digitally, encrypted, and then shredded. Participants will be fully informed about the study's objectives, procedures, and confidentiality measures, and their written consent will be obtained prior to screening and study enrolment, and re-confirmed orally before each testing session. In the event of cognitive impairment, participants will be presumed to have the capacity to consent unless demonstrated otherwise, in line with the Assisted Decision-Making Capacity Act (2015) Part 1, Section 3, Subsections 2–6105 and the HSE National Policy for Consent in Health and Social Care Research106.
Participants will have the right to withdraw at any time without consequence. In case of distress, participants will be referred to appropriate clinical support, and any necessary actions, such as informing their GP, will be taken with their consent. Discontinuation criteria for withdrawing a participant from the trial (but not stopping the overall trial) will include: withdrawal of consent by the participant; any condition that the research team or sponsor determines may jeopardise the participant’s safety if she or he continues participating in the trial; ineligibility arising during the study (e.g., participant recovers cognitive function between screening and baseline assessments; or aphasia identified subsequent to screening); an adverse event which requires discontinuation from the trial (e.g., hospitalisation for disease progression; a new diagnosis that would affect cognition; mortality).
Furthermore, participants may access their data upon request until the point at which the data are permanently anonymised at the conclusion of the study. Full details of their rights are outlined in the PIL. In such cases, participants will also be offered the opportunity to discuss their test results with the clinical neuropsychologist. A comprehensive Data Management Plan will ensure proper data collection, storage, and retention procedures, with anonymised data deposited in open-access repositories upon study completion. Data will be securely stored on an RCSI server, and only authorised personnel will have access. The safety and well-being of participants are paramount, and all trial procedures have been designed with minimal risk and previously feasibility tested.
The results of this pilot trial will be disseminated through a comprehensive strategy aimed at maximising outreach and impact. Information will be shared through local patient and family support meetings organised by the Irish Heart Foundation (IHF), the principal stroke charitable organisation in Ireland, as well as national conferences such as the IHF annual stroke survivor conference. Findings will also be presented at major stroke conferences, such as the UK Stroke Forum and the European Stroke Organisation conference, and published in peer-reviewed journals. To ensure accessibility, open access strategies will be employed, with research materials, including protocols, datasets, and study findings, made available through HRB Open, the Open Science Framework, and the RCSI repository. This comprehensive dissemination plan will contribute to informing clinical practice, policy, and the field of cognitive rehabilitation for stroke survivors.
In conclusion, this research aims to evaluate, through a pilot RCT, the feasibility and preliminary effectiveness of the StrokeCog-R intervention for PSCI, addressing a significant gap in current stroke rehabilitation. Given that cognitive impairment is a frequent consequence of stroke, this pilot trial will generate essential data on recruitment, retention, preliminary efficacy, and cost-effectiveness, to guide a future definitive trial. The potential benefits extend to patients, carers, healthcare providers, and policy-makers by offering an evidence-based approach to cognitive rehabilitation, improving patient outcomes, and reducing long-term healthcare costs associated with cognitive decline and dementia. Ultimately, integrating cognitive rehabilitation into routine post-stroke care could transform stroke rehabilitation practices, enhancing quality of life for stroke survivors and yielding lasting societal benefits.
Ethical approval was approved by Beaumont Hospital research ethics approval (REC reference: 23/14). Patients expressing interest in participation will provide written informed consent for screening.
Open Science Framework: Extended data for StrokeCog-R: Protocol for a Pilot Randomised Controlled Trial Evaluating the Feasibility and Acceptability of a Novel Cognitive Rehabilitation Intervention for Post-Stroke Cognitive Impairment, https://doi.org/10.17605/OSF.IO/CMKHT107
This project contains the following underlying data:
Data is available under CC-By Attribution 4.0 International.
SPIRIT checklist https://doi.org/10.17605/OSF.IO/CMKHT107,
Trial Governance Composition, Roles and Responsibilities:
Coordinating centre: RCSI University of Medicine and Health Sciences
Principal Investigator:
Design and conduct of StrokeCog-R
Liaising with Sponsorship Office
Budget administration and contractual issues with recruitment centres
Preparation of protocol and revisions
Organising steering committee meetings
Publication of study findings
Chair of Trial Management Committee and Trial Steering Committee
Steering committee (SC)
All trial co-applicants will be steering committee members (see title page for members)
Agreement of final protocol
Regular meetings to review progress of trial and, if necessary, agree changes to the protocol to facilitate the smooth running of the study.
Trial Management Committee (TMC)
(Principal investigator, Post-Doctoral researcher, Research Assistants, Stroke Physician, Clinical Neuropsychologist, Trial Biostatistician)
Study planning
Trial data management and approval of trial data management and data analysis plans
Advice for lead investigators
Core Trial Management Group
(Principal investigator, Post-Doctoral researcher, Research Assistants )
Study planning
Weekly trial management meetings
Organisation of steering committee meetings
Manage correspondence with research ethics committee and data protection officers
Preparation of protocol and revisions
Randomisation
Data management and development of a trial data management plan
Data verification
Publication and presentation of study findings
Trial Manager
Creation and maintenance of trial data entry system and data entry
Data verification
Data Monitoring: Formal Committee:
This study is a pilot trial involving a relatively small number of patients and a low-risk intervention. As such, an a priori decision was taken at the funding application stage that the data monitoring and safety monitoring functions of the trial will be managed by the Trial Steering Committee. The data monitoring function will involve monitoring data emerging from the trial with a particular view to identifying any safety issues, including issues that may need to be brought to the attention of the Sponsor and the trial participants. As part of the safety monitoring aspect of this trial, other potential adverse events will be monitored and recorded, including health outcomes that may be unrelated to the trial, such as hospital admissions, or other health events such as a new clinical diagnosis, and mortality.
Protocol Version 1: 29/04/25
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
No
Are the datasets clearly presented in a useable and accessible format?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Occupational therapy and Rehabilitation
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 09 May 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with HRB Open Research
Already registered? Sign in
Submission to HRB Open Research is open to all HRB grantholders or people working on a HRB-funded/co-funded grant on or since 1 January 2017. Sign up for information about developments, publishing and publications from HRB Open Research.
We'll keep you updated on any major new updates to HRB Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)