Keywords
Mobile Applications, Lung neoplasms, Early detection of cancer, Cancer screening, Health Promotion, Realist Review
Lung cancer, the leading cause of cancer death worldwide, is often diagnosed at advanced stages leading to a poorer prognosis. Mobile health (mHealth) interventions, which are healthcare technology utilizing mobile or other wireless technology, promise enhanced early detection by optimising lung cancer screening (LCS) implementation. However, their efficacy across various patient demographics and the underlying mechanisms that influence LCS success remain unclear and underexplored.
To explore the efficacy of mHealth interventions in promoting LCS uptake, focusing on patient demographics, intervention characteristics, and the underlying mechanisms and contexts influencing their effectiveness.
This realist review will employ an iterative literature search in databases such as PubMed, Scopus, Web of Science, and Embase. Selected studies will be assessed for relevance and rigour, extracting data on mHealth features, patient demographics, and intervention outcomes. Data will be analysed thematically to describe relationships between intervention mechanisms, contexts, and outcomes. Additionally, engagement from key stakeholders, including health experts and patients, will be sought during the synthesis phase.
This review aims to offer a comprehensive understanding of how and why mHealth interventions can influence LCS uptake and be effective across different patient demographics. These findings will provide insights into optimising mHealth interventions for LCS, potentially leading to earlier detections and improved patient outcomes.
Mobile Applications, Lung neoplasms, Early detection of cancer, Cancer screening, Health Promotion, Realist Review
Introduction references updated to include those relevant to inequalities in Lung Cancer Screening participation.
The paragraph regarding mHealth and Lung Cancer Screening has been refined, with a more targeted argument for this work.
An earlier stated rationale behind choice of review methodology in addition to relevant references.
Further information has been provided outlining the nature and expertise of our stakeholders and collaborators.
The strengths and limitations section has been updated to outline implications.
The methods section of the manuscript has been updated to explain in more detail the quality appraisal process as well as the CMO configuration extraction process.
Two new authors added, both of which have been involved in project administration, review, and editing of the paper.
See the authors' detailed response to the review by Jacqueline H Boudreau
See the authors' detailed response to the review by Sushant Patkar
With 2.2 million new cases and 1.8 million deaths globally per year, lung cancer is the second most common cancer and the leading cause of cancer death worldwide (Sung et al., 2021). A significant reason for its high mortality is delayed diagnosis, with lung cancer commonly diagnosed at an advanced stage (Walter et al., 2015). This results in a poorer prognosis, with lung cancer in the UK having a 1-year survival rate of 85% for Stage I disease versus just 25% for Stage IV disease (“NLCA annual report,” 2022).
Later stage lung cancer diagnosis is multifaceted due to disease, patient, and provider related factors. Symptoms particular to lung cancer may be vague in their presentation and not directly reflect chest or lung symptoms (e.g., fatigue and weight loss). Asymptomatic early lung cancer disease can contribute to delayed diagnoses and more advanced symptomatic disease at presentation (Walter et al., 2015). More than two thirds of lung cancer is diagnosed at stage three or four (McPhail et al., 2015). Delayed patient presentation may occur if the patient is unaware of the potential significance of their symptoms, misinterprets them, or is fearful of the consequences of a diagnosis (Cassim et al., 2019; Weller et al., 2019). Healthcare related factors include misdiagnosis or challenging diagnosis due to unclear symptoms and delays due to inefficiencies in healthcare systems (Guirado et al., 2022; Newman-Toker et al., 2020).
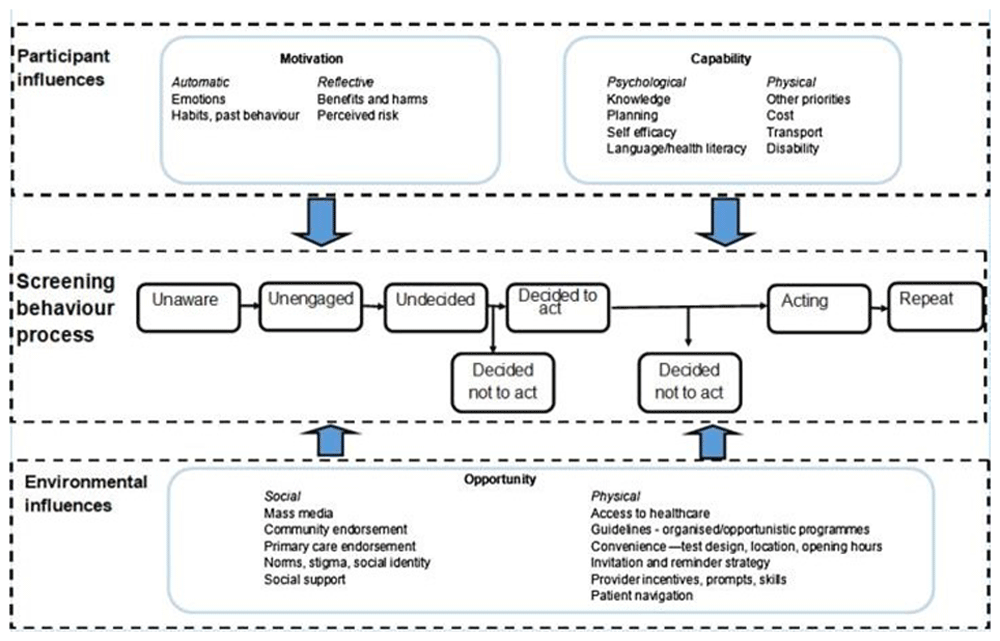
The UK National Screening Committee, the US Preventative Task Force, and the European Union position statements all currently recommend targeted lung cancer screening with low-dose computer tomography (LDCT) for those at high risk of lung cancer (Jonas et al., 2021; Mahase, 2023; Oudkerk et al., 2017). However, the success of screening uptake is compromised by uptake at various stages of the cancer screening pathway such as participant influences, screening behaviour processes, and environmental influences (Figure 1) (Robb, 2021). Fear, fatalism, and stigma may be present in at-risk communities (Quaife et al., 2018). Primary care providers may face difficulties in identifying and inviting at-risk individuals for further testing and examinations and making informed decisions with support from multidisciplinary teams. Furthermore, ethnic and regional inequalities persist, underscoring the likely need for tailored approaches to improve LCS participation (Boudreau et al.., 2021; Carter-Bawa, 2024).
The World Health Organisation defines mHealth as medical and public health practices supported by mobile and other wireless devices (WHO Guideline, 2019). With over 5.3 billion mobile phone users globally, mHealth provides unique ability to reach a great proportion of users instantaneously through voice, text (SMS), video and other multimedia services (Marcolino et al., 2018; Sereno et al., 2023). MHealth interventions encompass patient education, health behaviour change communication, data collection, provider training, etc. These interventions have demonstrated positive impacts across various diseases. For cancer specifically, mHealth has been utilised for self-care, self-management, and behaviour change among cancer survivors (Vaffis et al., 2023). However, notably in Lung Cancer specific apps – most are focused on treatment/survivorship, and none are integrated with existing health records.
Existing reviews suggest mHealth interventions can effectively boost breast, cervical, colorectal, prostate, or lung cancer screening uptake, knowledge, and awareness (Ruco et al., 2021). A variety of apps and interventions have been developed to support either cancer screening in general, such as the ePrognosis Cancer screening app (Kotwal & Walter, 2020), or specific types of cancer, such as GLAm for cervical cancer screening (Wanberg et al., 2023). A recent scoping meta-review found 67 mobile interventions, of which 57 (85%) targeted breast and cervical cancer awareness and screening uptake (Schliemann et al., 2022). These mHealth interventions were found to increase cancer screening uptake, most commonly through SMS and telephone calls. Dhar et al. identified that factors such as guided supervision, personalised suggestions, and theoretical intervention foundations can enhance adherence and efficacy of mHealth interventions in cancer care management (Dhar et al., 2023). Ardito highlighted the importance of multistakeholder co-design and testing of mHealth interventions, as well as addressing patient needs as a key incentive for mHealth use (Ardito et al., 2023).
The evidence for mHealth to support LCS is less developed (Table 1). A systematic review on mobile health in cancer screening reported only one study out of 23 examining a smartphone application for lung cancer screening (Salmani et al., 2020). This study, by Szanto et al., described the development of a smartphone application that assessed lung cancer risk and directed high-risk individuals to screening centres based on their geographic location. This application was downloaded by almost 14,000 users, indicating the potential reach and usefulness of this approach (Szanto et al., 2017). Ahern et al. deployed the Lung Age app to support risk assessments for chronic obstructive pulmonary disease (COPD) in a primary care setting. Participants found the app “intuitive and easy to use”, suggesting the viability of such technology in primary care populations who would likely form the target population for lung cancer screening (Ahern et al., 2016). This application was downloaded by a large number of users, indicating the potential reach and usefulness of this approach.
| Risk Assessment Tool | Variables Considered | Notable Features |
|---|---|---|
| PLCOm2012 (National Lung Screening Trial Research Team et al., 2011) | Age, race, smoking history, personal health history | Widely validated; used in US-based studies |
| LLP (Liverpool Lung Project) (Marcus et al., 2015) | Age, smoking duration, personal/family history of cancer | Developed for the UK population |
| NLST Criteria (Tammemägi et al., 2013) | Age, smoking pack-years, years since quitting | Criteria from the National Lung Screening Trial |
| Bach Model (Hoggart et al., 2012) | Age, smoking history, exposure to asbestos, personal/family history of cancer | Considers occupational exposure |
| ELCAP (Early Lung Cancer Action Project) (Henschke et al., 1999) | Age, smoking history | Simplified criteria; focuses on early detection |
Given the significance of early lung cancer detection and the rapid expansion of mHealth, a deeper understanding of the mechanisms underpinning its effectiveness in promoting LCS is needed. This synthesis will enhance current knowledge on patient demographics, intervention efficacy, and underlying mechanisms.
The Realist Review is a theory-driven approach to evidence synthesis that asks what works, for whom, in what contexts, and why. This methodology can address the shortcomings of many traditional review methods, moving beyond the efficacy of an intervention, ultimately providing explanatory models. This synthesis method values the relevance, richness, and rigour of data over traditional hierarchies of evidence. Ultimately, this review will seek to inform future attempts to design and implement mHealth interventions to enhance lung cancer screening engagement.
The aim of this review is to explore the associations between patient demographics, characteristics of mHealth interventions, and the underlying mechanisms and contexts that influence the efficacy of eHealth interventions in promoting lung cancer screening (LCS).
Specific objectives include:
1. To identify the patient demographic factors that influence the efficacy of mHealth interventions in promoting LCS.
2. To determine the specific characteristics of mHealth interventions that are effective across different demographic groups.
3. To discern the underlying mechanisms, theories, beliefs, and contexts that influence the effectiveness of mHealth interventions in promoting LCS.
Adhering to the RAMESES publication standards for realist syntheses, this study will utilise a realist synthesis approach to comprehensively examine the interplay between mHealth interventions and their efficacy in promoting lung cancer screening (LCS) (Wong et al., 2013). Synthesis of study data will focus on extracting data relevant to the underlying mechanisms, theories, contexts, and factors that determine the success of the interventions in question (Duddy & Wong, 2023).
We will report the findings in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021).
An initial exploratory scoping of the literature will be undertaken to identify key theories, concepts, and existing evidence related to mHealth interventions in LCS.
Development of search terms. To ensure a comprehensive and systematic retrieval of relevant literature, our search strategy is designed with a combination of subject heading terms, such as Medical Subject Headings (MeSH), and relevant free-text keywords. These terms and keywords identified are based on preliminary scans of the literature, consultation with stakeholders in the field (as outlined below), and alignment with our review objectives.
Themes and keywords. Our search strategy will be focusing on three different terms:
1. Mhealth term including terms such as: ‘digital interventions’, ‘phone apps’, or ‘phone interventions’
2. Lung cancer term including: ‘lung carcinoma’, or ‘pulmonary neoplasm’
3. Screening terms such as: ‘early detection’ or ‘new diagnosis’
To see a sample search strategy, please see extended data.
Database search. We will systematically search the following electronic databases: Cochrane Library, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Embase, MEDLINE, ProQuest Dissertation and Thesis Database, Scopus, Web of Science, and PsychInfo.
Search strategy refinement. To ensure maximum yield of relevant articles, we have included an information specialist on our authorship team (KW). This specialist will help refine our search terms, ensure appropriate Boolean operators are applied, and adapt the strategy to the specific requirements of each database.
Additional searches. Beyond the database searches, we will also manually search the reference lists of included studies and relevant reviews to ensure that all pertinent articles are captured. Grey literature, such as conference abstracts and reports, will also be considered to reduce publication bias.
Inclusion criteria: articles focusing on lung cancer screening interventions, published in English language. Exclusion criteria includes non-peer reviewed studies and interventions focusing on cancer outcome or management. Two authors will independently screen the title and abstract of all papers for relevance and rigour. Discrepancies will be discussed and resolved through consensus. Reviewers will then independently assess the full text of potentially relevant studies to determine whether they meet the inclusion criteria. In cases of uncertainty or disagreement, a third reviewer's opinion will be sought.
All search results will be imported into a reference management software (www.Rayyan.ai) (Please also refer to https://systematicreviewsjournal.biomedcentral.com/articles/10.1186/s13643-016-0384-4) to organise and screen titles and abstracts and remove duplicates (Ouzzani et al., 2016). This software will also facilitate the tracking of the selection process, which will be depicted in a PRISMA flow chart in the final review.
Data will be extracted from included studies by one reviewer and confirmed by a second reviewer using a pro forma specifically designed for the purpose. The extracted data will focus on mHealth features, patient demographics, intervention outcomes, and any context or mechanism information related to the efficacy of the intervention. To see an example form for data extraction, please see extended data.
Realist synthesis adopts a theory-driven approach to evidence synthesis. The process begins with the articulation of initial program theories that explain how mHealth interventions might work, for whom, and under what circumstances. These theories are then iteratively tested and refined against the evidence gathered from the included studies.
1. Initial program theories: Drawing from the literature and stakeholder consultations, we will propose tentative theories that suggest how mHealth interventions lead to their observed outcomes. These theories will elucidate the mechanisms that drive these outcomes and the contexts in which these mechanisms are triggered.
2. Testing and refining theories: As we gather evidence from the included studies, we will test the initial theories against this empirical data. This iterative process will involve:
• Context-mechanism-outcome (CMO) configuration: Central to realist synthesis, we will identify and analyse any intervention contexts such as the social-economical-demographics of participants, psychological state and readiness to undertake the intervention, and the setting or modality of the intervention. Mechanisms are triggered by contexts, which lead to the outcomes of interest. Possible mechanisms include responses of participants and ease of access of interventions. Outcomes can include uptake, useability, health-related outcomes, and any others related to the intervention (De Souza, 2013; De Weger et al., 2020).
• Documenting Contradictions and Variations: Any variations in observed outcomes will be documented, and we will seek to explain these variations based on different contexts and mechanisms.
3. Synthesising Evidence:
• CMOs will be coded thematically based on emerging themes as the review progresses. Duplicates will be removed. CMOs will undergo theory mapping to theoretical frameworks such as the COM-B and/or I-SAM models (Michie et al., 2011).
• Themes and patterns will be identified that provide insights into when, why, and how mHealth interventions are effective (or ineffective) in promoting LCS across different patient demographics. This synthesis will result in refined program theories that offer a nuanced understanding of mHealth interventions.
Realist Reviews can address the shortcomings of many traditional review methods. A realist review offers a method by which to provide explanatory models. Unlike traditional review methods that apply a strict criteria for evidence appraisal and assessment of bias, the approach to quality appraisal in realist reviews must be rooted in the contribution of evidence to the programme theory being developed or tested. This criteria for the appraisal of evidence should encompass Relevance, Rigor, and Richness (Dada et al., 2023). To this end, we will create a tool assessing the following:
1. Relevance: Does the study provide data on the contexts, mechanisms, or outcomes of interest?
2. Richness: To what degree does it contribute to theory development?
3. Rigor: Was the study designed and conducted in a way that provides credible insights into the CMO configurations?
Data synthesis. Given the narrative and theory-driven nature of realist syntheses; traditional meta-analysis might not be directly applicable. However, when the data allows, we may undertake:
1. Narrative synthesis of quantitative and qualitative data: This involves summarising the findings from different studies to provide an overview of the evidence landscape.
2. Comparative analysis: If there are studies with comparable data points, comparative analyses might be conducted to identify patterns or trends across them.
3. Addressing heterogeneity: if significant heterogeneity exists in the findings, this will be explored qualitatively to understand the different contexts and mechanisms that might explain the variations between studies.
In line with realist methodology, key stakeholders, such as health experts in psychology, epidemiology, and public health, patients, and Information and Communication Technology (ICT) professionals, will be engaged during various phases of the synthesis. Their insights will provide valuable contextual understanding and will be instrumental in refining the program theories.
In line with our objectives, this realist synthesis seeks to explore and understand the relationship between patient demographics, beliefs, mHealth interventions, and underlying mechanisms influencing the efficacy of eHealth interventions in promoting lung cancer screening (LCS). By analysing the existing literature and synthesising key insights, we aim to provide a comprehensive overview of the factors that determine the success or failure of mHealth interventions in promoting LCS.
The strength of this review lies in its realist approach, which moves beyond assessing mere efficacy to unpacking the 'why', 'when', and 'how' of mHealth interventions (Rycroft-Malone et al., 2012; Wong, 2018). By doing so, we hope to understand and explain the mechanisms and contexts that shape outcomes, and to ultimately inform the development of LCS mhealth tools supporting engagement.
There are several limitations to our proposed approach. First, the dynamic nature of mHealth technology and its rapid evolution might mean that some interventions become outdated quickly. Second, cultural, geographical, and infrastructural differences may influence the generalisability of findings across different settings. Finally, the inherent complexity of realist syntheses, which draw from a wide range of sources and types of evidence, might introduce challenges in synthesising and interpreting findings.
While several studies and reviews have explored the potential of mHealth in various healthcare domains, few have specifically focused on its role in lung cancer screening (Schliemann et al., 2022). Preliminary scoping indicates that while there is growing interest in this area, comprehensive syntheses that integrate the nuances of patient demographics, intervention characteristics, and contextual factors are lacking.
The findings from this synthesis have several implications. For researchers, it underlines the importance of considering the holistic context in which mHealth interventions are deployed. For policymakers and practitioners, understanding the mechanisms driving successful interventions can inform the design of future policies and national programmes. There is also potential in integrating mHealth data with other digital health platforms to provide a more comprehensive patient profile, aiding in risk assessment and early detection (Najjar, 2024; Ogundaini et al., 2021).
Ultimately understanding the successful implementation of mHealth interventions can guide clinicians in recommending appropriate tools to patients (Hamberger et al., 2022), and supporting policymakers to design guidelines that promote effective mHealth strategies in lung cancer screening (Barkman & Weinehall, 2017).
The design, interpretation, and dissemination of our research will be done in consultation with the PRiCAN PPIE group as well as our patient co-author Mr Seamus Cotter – Chairperson of the Irish Lung Cancer Community.
Research Ethics Committee approval is not required for this article.
No data is associated with this article.
OSF Repository: [Dataset] mhealth protocol for “Protocol for a Realist Review of mHealth in Lung Cancer Screening: Understanding Mechanisms, Contexts, and Intervention Characteristics for Enhanced Participation”.
DOI:10.17605/OSF.IO/4AXZB. Gong (2024)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0) (https://creativecommons.org/licenses/by/4.0/).
The authors would like to acknowledge Irsoon Hassan (RCSI University of Medicine and Health Sciences) for her work on a pilot search strategy and early version of the protocol.
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: genomics, precision oncology, lung cancer, artificial intelligence
Is the rationale for, and objectives of, the study clearly described?
Partly
Is the study design appropriate for the research question?
Partly
Are sufficient details of the methods provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Not applicable
References
1. Boudreau JH, Miller DR, Qian S, Nunez ER, et al.: Access to Lung Cancer Screening in the Veterans Health Administration: Does Geographic Distribution Match Need in the Population?. Chest. 2021; 160 (1): 358-367 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: lung cancer screening, mixed methods research, systematic reviews
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 05 Dec 25 |
||
|
Version 1 24 Jan 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with HRB Open Research
Already registered? Sign in
Submission to HRB Open Research is open to all HRB grantholders or people working on a HRB-funded/co-funded grant on or since 1 January 2017. Sign up for information about developments, publishing and publications from HRB Open Research.
We'll keep you updated on any major new updates to HRB Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)