Keywords
Health Economic Analysis plan, Cost-effectiveness analysis, Dementia, Sensory care
This article is included in the Dementia Trials Ireland (DTI) and Dementia Research Network Ireland (DRNI) gateway.
Sensory challenges exacerbate the dementia symptoms of nursing home residents. The Sense-Cog Care trial is piloting a multi-faceted intervention to investigate whether optimising and supporting hearing, vision, and sensory-friendly environments through the “sensory champion” model in Irish nursing homes can improve quality of life and dementia-related outcomes and be cost-effective (Connelly et al., 2023).
A Health Economic Analysis Plan (HEAP) provides a standardised and comprehensive framework by outlining the systematic approach, methodologies, and key considerations involved in assessing the cost-effectiveness of a healthcare intervention. The HEAP presented in this paper describes the alongside-trial decision modelling approach used for preliminary estimates of cost-effectiveness and to inform the design of the final intervention.
A Markov model will be used to simulate residents' health progression with and without the intervention. Costs will be estimated from the Irish health and social care perspective. A key output of the planned economic analysis will be the Incremental-Cost-Effectiveness Ratio (ICER) which describes the cost over one additional unit of Quality-Adjusted Life Year (QALY). A Probabilistic Sensitivity Analysis (PSA) will be conducted in the form of a Monte Carlo simulation to examine parameter uncertainty and the probability of a sensory champion being cost-effective.
Tables for inputs and outputs are presented, and graphical representations for uncertainty, such as a tornado plot and a Cost-Effectiveness Analysis Curve (CEAC), will be produced. The results will be interpreted in the context of the Irish cost-effectiveness thresholds of €20,000 and €45,000 per QALY.
To our knowledge, this is the first study to model the cost-effectiveness of a sensory intervention for nursing home residents with dementia. We aim to develop an early-stage Bayesian model that serves as a prior for further research.
Health Economic Analysis plan, Cost-effectiveness analysis, Dementia, Sensory care
In Ireland, around 64,000 individuals are currently living with dementia, with around 34% residing in long-term care facilities. Projections indicate that this number will more than double, reaching over 150,000 by the year 2045 (HSE & National dementia office, 2020).
The Irish National Survey of Dementia in Long-Term Residential Care revealed that approximately 68% of residents in long-term care facilities are living with dementia (Cahill et al., 2014). The survey found that in specialised dementia care units, only about 50% of Long-term care staff and healthcare assistants (HCAs) had received training in dementia care and estimated that only 10% of residents’ symptoms are directly caused by dementia, while the remaining 90% result from the quality of care they receive in inappropriate settings (Cahill et al., 2014). Staff training is essential for managing the complex needs of dementia patients and improving their overall well-being, thereby enhancing their quality of life (Cahill et al., 2014).
Sensory impairments, including hearing and vision loss, are common among these residents, exacerbating cognitive decline and negatively impacting their quality of life (Amieva et al., 2015; Lin et al., 2013; Yeo et al., 2023) The Lancet Commission on dementia prevention identified hearing loss as the highest individual risk factor for developing dementia, with a relative risk of 1.09 (Livingston et al., 2020). Despite this, hearing aid use remains low. The Irish study of longitudinal ageing (TILDA) found that hearing loss is prevalent among older adults in Ireland, affecting 58% of men and 54% of women over the age of 75, yet only 11% of this population uses hearing aids (McGarrigle & Donoghue, 2023). The report linked the underutilisation to poorer mental health, increased loneliness and depression, and lower quality of life, which could be improved through better screening and provision of hearing aids.
The most reported reason for poor use of hearing aids/glasses is related to a lack of maintenance and care procedures and poor adherence support (Leroi et al., 2021). A study in the UK by Cross et al. (2024) reported that only 26.7% of long-term care staff regularly checked or tested hearing aids, providing sensory care to just half of those who needed it. Similarly, the provision of sensory assisting devices such as hearing aids or glasses is often overlooked, contributing to serious comorbidities arising from untreated sensory loss. This oversight is further exacerbated by suboptimal environmental conditions, including loud TVs, inadequate lighting, and poor surface design (Leroi et al., 2021).
The SENSE-Cog Care trial aims to address these issues by implementing a multi-faceted intervention in Irish long-term care homes and is currently conducting a feasibility trial. The intervention will include hearing and vision assessments and the provision of devices for the correction of sensory impairment. It will also include staff training and the election of staff members as ‘sensory champions’ who will receive further training in cognitive health and sensory cognitive support. The intervention will also focus on creating sensory-friendly environments and optimise care pathways to hearing/vision care providers. (Connelly et al., 2023). The trial aims to evaluate the intervention’s impact on key outcomes for residents with dementia and includes a health economics component (Connelly et al., 2023).
In a resource-constrained healthcare system, efficient allocation of limited resources is an important factor in policy decisions, and it has become more common to incorporate economic evaluations into randomised controlled trials (Drummond et al., 2015). Health economics aids these decisions by evaluating the cost-effectiveness of healthcare interventions. An economic analysis weighs the additional value of a particular technology against the opportunity cost of foregoing something else that the health budget could have generated, aiming for efficient allocation to maximise health or welfare outcomes (Briggs et al., 2006).
Writing a Health Economic Analysis Plan is recommended practice in alignment with agreed international guidelines with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist (Husereau et al., 2024), which requires a Health Economic Analysis Plan to be written and made available prior to publication. A Health Economic Analysis plan establishes the strategy and the methods for the cost-effectiveness analysis, promoting transparency and establishing guidelines and structure for economic assessment. Thorn et al. (2021) conducted an Expert Delphi Consensus Survey on the content of Health Economics Analysis Plans (HEAPs) for trial-based economic evaluations. Based on this content, items for the model-based HEAP were selected, omitting those specific to trial-based evaluations.
This Health Economic Analysis Plan (HEAP) describes the alongside-trial model-based economic analysis used to produce initial estimates of cost-effectiveness based on available evidence. It was created following the guidelines for the Economic Evaluation of Health Technologies in Ireland from the Health Information and Quality Authority (HIQA, 2016). The methodologies selected align closely with those endorsed by reputable entities such as the National Institute for Health and Care Excellence (NICE) and are heavily inspired by seminal works such as the handbook "Decision Modelling for Health Economic Evaluation" by Briggs, Claxton, and Sculpher (2006).
This research aims to contribute to bridging the gap in economic evaluation of non-pharmaceutical interventions in long-term care home populations. As a preliminary assessment, it provides structured estimates of costs and outcomes associated with the intervention, informing the design of the final intervention, and laying the foundation for future research and discussions.
The planned economic evaluation will use a purpose-built, Excel-based Markov model to simulate the progression of dementia of long-term care home residents with various sensory conditions. The model will consider the prevalence of the various sensory conditions in the treatment-arm long-term care homes, which receive the SENSE-Cog Care sensory champion intervention, and control arm long-term care homes, which provide care-as-usual.
The extent to which the intervention reduces the prevalence of unaddressed sensory impairment will be modelled and the uncertainty reflected in sensitivity analysis, as well as its impact on the progression of dementia of the residents and their quality of life.
The model will estimate all the benefits and costs of each arm using a lifetime horizon, which means that all future costs and benefits will be considered, and an Incremental Cost-effectiveness Ratio (ICER) will be produced. ICER is defined as the difference in the costs divided by the difference in the effectiveness of alternative courses of action (Drummond et al., 2015). In this case, the comparison will be between the implementation of the SENSE-Cog Care intervention and continuing with care as usual. The ICER essentially quantifies the cost required to generate one additional unit of Quality-Adjusted Life Year (QALY), which provides a measure of the intervention’s value in terms of health outcomes. This figure can then be compared against the willingness-to-pay (WTP) threshold in Ireland, which is typically considered to be between €20,000 and €45,000 per QALY (O’Mahony & Coughlan, 2016). The WTP threshold represents the maximum amount that society is willing to pay for a QALY, providing a benchmark to determine whether the intervention is considered cost-effective (Drummond et al., 2015). The ICER will be calculated using the formula below:
, where describes the total costs of the control group (C), describes the total costs of the treatment group (S) that receives the sensory care intervention, is the sum of QALYs of the control group, is the sum of QALYs of the treatment group.
The model will consider the long-term care home residents with dementia with four categories (i) of sensory impairment: residents with no sensory impairment (1), residents with unaddressed hearing impairment (2), residents with unaddressed vision impairment (3) and residents with unaddressed dual impairment (4).
The sensory conditions are each assumed to have a different progression of dementia and to have an impact on health state related quality of life. This leads to differences in lifetime QALY’s and costs of residents in each category. They will be weighted (W%) according to their prevalence in the treatment and control arm nursing homes.
In summary, the planned CEA simulation will estimate the incremental costs and benefits of the intervention by examining how effectively it reduces the prevalence of unaddressed sensory impairments, which can impact the progression of dementia and improve the quality of life for residents in Irish long-term care homes.
The Markov model (see Figure 1) will have four states: mild, moderate and severe stages of dementia, and a fourth absorbing state which is death. A one-way model of dementia is used where returning to a less severe state is not possible.
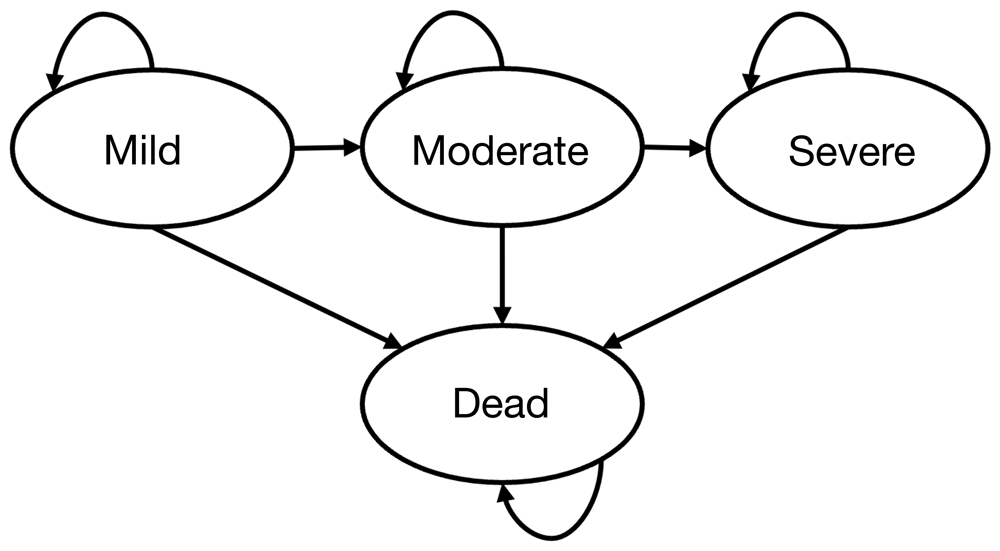
Figure 1 illustrates the key health states (represented by ovals) related to stages of dementia and transitions between these states (represented by arrows) indicating the likelihood of staying or moving between stages (Mild, Moderate and Severe) or the likelihood of death (an absorbing state) from each state.
The findings from Effective interventions for potentially modifiable risk factors for late-onset dementia: A costs and cost-effectiveness modelling study by Mukadam et al. (2020) will be used to determine transition probabilities and utilities. In this model, the probabilities will be adjusted by relative risk factors to capture the potential accelerated impact of sensory impairments on dementia progression. This will be done by multiplying the probabilities by a relative risk variable (RR) greater than 1 to account for the potential accelerated impact of poor sensory health on the natural progression of the disease (see Table 9 and Table 6). Population death rates will be imported from Irish data and will affect the transition probabilities according to age and sex (see Table 4).
The model cycle length will be quarterly, as the SENSE-Cog care will also conduct their follow-up quarterly. This cycle length also takes into account the small probability of progressing through multiple health states in a single year. The horizon will be a lifetime horizon to capture all the costs and health benefits of the intervention. This will be set at 10 years, which is 40 cycles.
The direct costs of the intervention, which are hypothetical since the model-based evaluation precedes the trial, along with the cost consequences, will be considered from an Irish health and social care perspective.
Direct costs will include a sensory champion cost, which considers any training-related staff costs of the intervention, purchases of hearing aids and glasses, which will be estimated based on the market price of these devices in Ireland, and hearing and vision screening costs, which will be estimated based on provider quotes (see Table 2). For cost consequences, the weekly institutional cost of care, along with medical, community, and informal care costs, will be considered (see Table 3). These will be estimated based on the best available evidence.
Costs and QALYs will be discounted at 4% to present value as per HIQA recommendations (HIQA, 2016). This reflects a societal preference for immediate benefits over future benefits and is an approach to express results in present value often found in guidelines, such as those by NICE (YHEC, 2016). Inflation is adjusted using the Irish Consumer Price Index (CPI) to standardize all costs to the equivalent value in 2024 euros.
Quality Adjusted Life Year (QALY) will be the effectiveness outcome value as recommended by HIQA. It captures an individual’s health-related quality of life and length of life and allows for comparability of economic evidence (Drummond et al., 2015). The QALY is calculated by multiplying a utility value by the duration spent in that state (e.g., one year in a health state with a utility value of 0.5 equates to 0.5 QALYs). Utility values measure preference for a health state, ranging from 0.0 (death) to 1.0 (perfect health) (Drummond et al., 2015).
The CEA will incorporate utility values from the study by Mukadam et al. (2020) for the health states of mild, moderate, and severe dementia (see Table 5). In the model, changes to the progression of dementia influence QALYs. For example, slowing down dementia progression results in fewer residents spending time in more severe, lower-utility states, thus increasing overall QALYs by extending time spent in higher-utility states.
For simplicity, sensory health will be categorised as either no impairment or unaddressed impairment, and its impact on utility will be similarly binary. The model incorporates the impact of sensory impairment on utility values by modelling it as a binary variable. It either describes normal utility or lower utility due to sensory impairment (see Formula 13 & Formula 14). This approach simplifies the parameter by creating an overall estimate of changes in health-related quality of life due to unaddressed sensory impairments.
Assessing the prevalence of unaddressed sensory impairment before and after the intervention is a key assessment in evaluating the potential cost-effectiveness of the treatment.
The weights are determined based on the probability of a resident being in a particular category, considering both the presence of sensory impairment and whether it is addressed. The model considers the progression of dementia and associated health-state utilities to be the same for residents who have addressed their impairments as for those with no impairments, treating both groups as having ‘no unaddressed impairment’. Figure 2 shows a flow diagram that represents the part of the model that determines these weights.
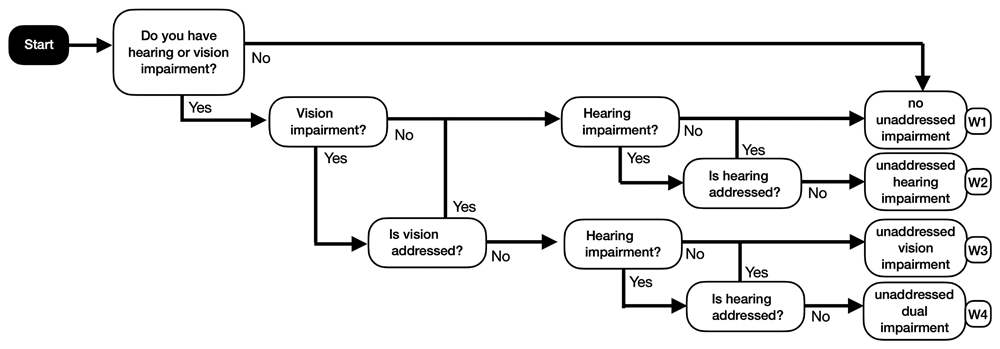
The flow chart determines the prevalence of dual impairment, hearing impairment, vision impairment and no impairment, depending on the answers given. Questions are represented by ovals; answers are indicated with arrows. If a resident has both vision and hearing impairments and neither is addressed, they are categorised as having unaddressed dual impairment. Addressing either hearing or vision impairment or having no impairment at all, classifies an individual as having no unaddressed impairment. Therefore, if a resident has unaddressed vision impairment but either does not have hearing impairment or has addressed hearing impairment, they are considered vision impaired only. Conversely, if a resident has unaddressed hearing impairment but either does not have vision impairment or has addressed vision impairment, they are categorised as having unaddressed hearing impairment.
Whether a sensory impairment is addressed or unaddressed depends on whether the impairment can be corrected with glasses or hearing aid, if the resident owns these devices, and if they use them (also referred to as adherence in the literature [Leroi et al., 2021]). The flowcharts in Figure 3 and Figure 4 illustrate how the model incorporates these considerations, by breaking down further the Is hearing addressed- and Is vision addressed ovals in figure 1.
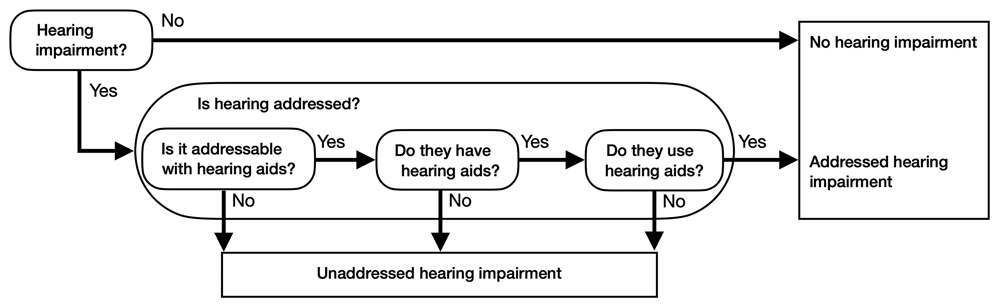
The flow chart determines how much of hearing impairment is addressed or unaddressed depending on the answers given. Questions are represented by ovals; answers are indicated with arrows.
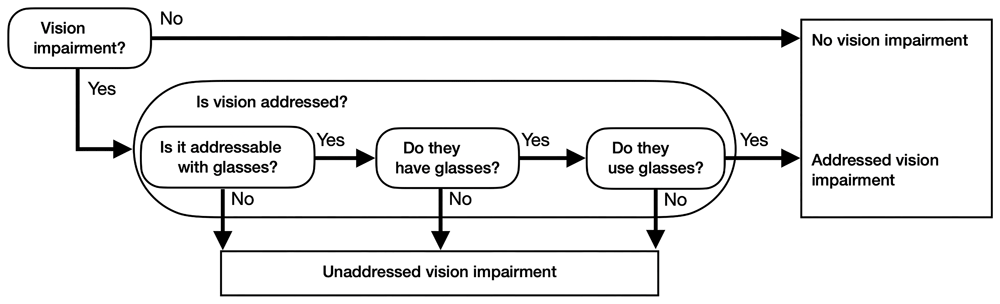
The flow chart determines how much of vision impairment is addressed or unaddressed depending on the answers given. Questions are represented by ovals; answers are indicated with arrows.
Since the planned CEA will be conducted before the trial, the effects on the proportion of residents with glasses and hearing aids, and their usage, will be hypothetical (see Table 8 and Table 7). The simulation will assume that all residents will be screened, and those with addressable impairments in the intervention group will accept these devices. Due to the novelty of the intervention and uncertainty about its effectiveness in overcoming barriers to device use, the simulation will run different levels of adherence improvement—10%, 30%, and 70%—to assess the potential impact on the ICER.
The planned cost-effectiveness simulation results will be influenced by both the intervention’s ability to mitigate unaddressed sensory impairment, and the extent to which sensory impairment impacts Costs and QALY of residents with dementia in long-term care homes.
To conduct probabilistic sensitivity analysis (PSA) a Monte Carlo simulation is conducted to explore uncertainties in our model's input variables. Monte Carlo simulation treats model parameters as random variables, using probabilistic distributions to generate 1,000 ICER results by randomly sampling from these distributions. As recommended in Decision Modelling for Health Economics (Briggs et al., 2006) costs will be assumed to have a gamma distribution, utilities and transition probabilities will be distributed along a beta distribution, and the relative risk parameter will be assigned a lognormal distribution. Where the standard deviation is not known a 20% standard deviation is applied, with a 10% standard deviation being used for the utilities and transition probabilities from the Mukadam et al. (2020) study.
Scenario analysis will be performed on the treatment effect of improvement in the share of sensory aids used by those who have them, by +10%, +30%, and +70% (see Formula 23 to Formula 28).
In the absence of robust evidence, the analysis will vary key uncertain parameters by several orders of magnitude to assess their influence on the incremental cost-effectiveness ratio (ICER), without attempting to determine the most probable scenario.
Since this is a plan, no results have been generated yet. Table 1 shows the expected presentation of results based on the methods described in this document.
| Item description | Unit cost (€) | Source |
|---|---|---|
| Sensory champion cost | x | x |
| Hearing aid cost | x | x |
| Glasses cost | x | x |
| Hearing aid screening cost | x | x |
| Glasses screening cost | x | x |
| Age | Male | Female |
|---|---|---|
| nt | ||
| nt+1 | x | x |
| nt+2 | x | x |
| nt+3 | x | x |
| nt+4 | x | x |
| nt+5 | x | x |
| nt+6 | x | x |
| nt+7 | x | x |
| nt+8 | x | x |
| nt+9 | x | x |
| nt+10 | x | x |
| Item description | Value | Source |
|---|---|---|
| RR unaddressed hearing loss | x | x |
| RR unaddressed vision loss | x | x |
| RR unaddressed dual impairment | x | x |
| Input item | P(%) | Source |
|---|---|---|
| PS(uses glasses) | x | Formula 23, Formula 25 & Formula 27 |
| PS(uses hearing aids) | x | Formula 24, Formula 26 & Formula 28 |
| PS (has glasses) | x | Formula 29 |
| PS(has hearing aids) | x | Formula 30 |
| From/To | mild | moderate | severe | death |
|---|---|---|---|---|
| mild | 1 – X * RRi – pop rate | X * RRi | 0 | pop rate |
| moderate | - | 1 – Y * RRi – Z – pop rate | Y * RRi | Z + pop rate |
| severe | - | - | 1 – Z – pop rate | Z + pop rate |
| death | - | - | - | 1 |
Pop-rate stands for yearly population death rate and RRi stands for relative risk for each category of impairment i. X is the transition probability from mild to moderate. Y is the transition probability from moderate to severe. Z is the increased probability of death due to dementia. Adapted from Effective interventions for potentially modifiable risk factors for late-onset dementia: a costs and cost-effectiveness modelling study, by Mukadam N, Anderson R, Knapp M, et al Lancet Healthy Longev 2020 with modifications by the author.
Deterministic scenario analysis will demonstrate changes to the ICER based on improvements (of 10%, 30% and 70%) in the rate of use of hearing aids and glasses for residents who have them. Multiple tables will be produced if orders of magnitude analysis on key uncertain parameters is deemed necessary.
To represent uncertainty in our model, probabilistic sensitivity analysis (PSA) will be conducted in line with methods commonly used in health economic evaluations ability to depict uncertainty and the robustness of the model’s outcomes (Briggs et al., 2006; Drummond et al., 2015). Results from the PSA will be presented in a scatterplot, showing the distribution of incremental costs and Quality-Adjusted Life Years (QALYs) on the cost-effectiveness plane. This will allow for a visual representation of the uncertainty surrounding the Incremental Cost-Effectiveness Ratio (ICER). Additionally, a cost-effectiveness acceptability curve (CEAC) will be plotted to demonstrate the probability of the intervention being cost-effective at various willingness-to-pay thresholds. To further explore the sensitivity of the ICER to individual parameters, a tornado diagram will be generated, ranking parameters by their impact on the ICER, with the most influential variables displayed at the top and the least influential at the bottom. These three steps of sensitivity analysis will be repeated for each deterministic order of magnitude scenario analysis.
Publishing a HEAP promotes transparency and establishes a structure for the economic assessment. Establishing a similar consensus to Thorn et al. (2021) around model-based HEAPs would be beneficial.
This Health Economic Analysis Plan presents an approach to simulating the cost-effectiveness of sensory aids for residents with dementia in long-term care settings in Ireland. To our knowledge, this is the first study to model the cost-effectiveness of a sensory intervention for nursing home residents with dementia. We aim to develop an early-stage Bayesian model that serves as a prior for further research.
This is HEAP version 1. Any modifications to this plan will be documented and reported in subsequent HEAPs, if changes occur.
Reporting deviations: The following items from the Thorn et al. (2021) expert consensus on essential trial-based evaluation elements were excluded: Trial HEAP version, Trial protocol version, Trial SAP version, Trial design, Trial start and end dates, data cleaning for analysis, sampling uncertainty and resource use data collected.
The above items from Thorn et al. (2021) expert consensus of essential items were omitted as they were specific to trial-based evaluations.
Patients and the public will not participate in the design, execution, reporting, or dissemination of the planned model-based economic evaluation. PPI is involved in the wider study.
To ensure full transparency and facilitate easy replication, the following sections provide a detailed description of the formulas, input variables, and the model’s process for generating the desired outputs. This thorough documentation also serves as a form of model validation, ensuring that the methodology is robust and can be independently verified by others.
Mathematically the Markov model can be described as the multiplication of two matrices. This approach is also described in the appendix to the study by Mukadam et al. (2020).
1. A matrix that contains the number of residents in each state at any time t:
is the starting distribution at t=0, which will be determined from our Irish long-term care home dataset.
2. And a transition probability matrix:
The distribution of residents at time t is given by the multiplication of the two matrices:
Treatment arm (S) Cost
, where the cost consequences for mild, moderate and severe states are calculated weekly and then adjusted to a quarterly basis by multiplying by 13. Similarly, the annual discount rate (dr) is divided by 4 for a quarterly discount rate.
Control arm (C) Cost
The formula for the control arm is the same, except it does not include the cost of the intervention:
Direct cost formula
, where P(n of new hearing aids) = PS (has hearing aids) – PC(has hearing aids) and stands for the number of new hearing aids purchased as part of the intervention and P(n of new glasses) = PS(has glasses) – PC(has glasses) which accounts for the number of new glasses purchased as part of the intervention.
Indirect cost formulas
The formula for QALY1, which is the QALY for non-impairment, is:
The formula for QALY2,3,4, which are the QALY’s for unaddressed sensory impairment, are:
, where β is a loss in health utility from sensory impairment.
Pk(HI = 1) is the notation that is used for the probability that an individual has an unaddressed hearing impairment, and Pk(VI = 1) is the notation used for the probability that an individual has unaddressed vision impairment in the treatment and control arm. Conversely, Pk(HI = 0) and Pk(VI = 0) represent the probabilities that an individual does not have an unaddressed hearing or vision impairment, respectively.
The probabilities for the weights of the model are the following:
The probabilities for having or not having a particular unaddressed sensory impairment are described below:
How the model assumes that all residents who can benefit from glasses and hearing aids will possess them in the intervention arm:
Considering not all residents will use the sensory aids due to obstacles identified in the literature, scenario analysis will be conducted on the rate of adherence for glasses PS(uses glasses) and rate of adherence for hearing aids PS(uses hearing aids) in the treatment arm:
Scenario for 10% increase
Scenario for 30% increase
Scenario for 70% increase
Ethical approval and consent were not required.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with HRB Open Research
Already registered? Sign in
Submission to HRB Open Research is open to all HRB grantholders or people working on a HRB-funded/co-funded grant on or since 1 January 2017. Sign up for information about developments, publishing and publications from HRB Open Research.
We'll keep you updated on any major new updates to HRB Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)