Keywords
costs, ageing, demography, policy, functional limitations, end of life, proximity to death
This article is included in the TILDA gateway.
This article is included in the Ageing Populations collection.
costs, ageing, demography, policy, functional limitations, end of life, proximity to death
Health care spending accounted for approximately 9% of gross domestic product in Organisation for Economic Co-Operation and Development (OECD) countries in 2019, the last complete year before the COVID-19 pandemic1. Resource allocation decisions in health care therefore have substantial impacts at the macroeconomic level, but also at the microeconomic level, where funding and availability of services may affect individual health, wealth and productivity2.
Health-related demands will always exceed available resources, placing a moral and practical imperative on decision-makers to fund those services that provide the best value3. This challenge, which economists frame in terms of ‘scarcity’, will be increasingly complex through the 21st century as populations age. Research has repeatedly shown that at the individual level the most important drivers of rising costs are not age per se, but instead, on the demand side, proximity to death, and, on the supply side, technology and staffing4,5. At the population level, costs will increase due to the rising total number of people living and dying with serious medical illness6, the demand for health care workers growing more quickly than supply7,8, and the number of medical technologies increasing persistently5,9–11.
Ireland is early in the demographic ageing process compared to other high-income countries12, but faces the same structural challenges and the same need to reform health care services for the population health needs and resource constraints of the 21st century13,14. A relatively young population today translates to faster-growing future needs: Ireland has the fastest ageing population in the European Union, with an expected three-fold increase in those aged 80+ years and near doubling of annual deaths in the next 20 years15. Compared to other western European nations, the Irish health care system has long been distinguished by a combination of relatively high per-capita spending and a relatively limited basket of entitlements under universal coverage16. Notable system characteristics include a reliance on acute inpatient hospital admissions given weak primary care capacity, high medications spending, non-compulsory insurance that improves access to some services for policyholders, and fast-growing population health needs16–18. Partly in recognition of these issues, Ireland has engaged in a wide-ranging health policy update since 2017, known as the ‘Sláintecare’ reforms, with mixed progress19–22.
High-quality data on individual-level health care costs in Ireland are essential to support monitoring, planning and evaluation of services, and the allocation of scarce resources to maximise public welfare. The lack of a unique patient identifier prevents researchers from using routine administrative data to estimate individual-level costs23. In 2021 researchers published the first standardised set of unit costs for non-acute health care in Ireland24, a long-awaited development and a critical step for health economics research in the state25.
The Irish Longitudinal Study on Ageing (TILDA) is a biennial study of people aged 50+ years in the Republic of Ireland that started in 2009–2011. Among many individual-level variables in a rich dataset, TILDA collects data on participants’ demographic, socioeconomic, early life, physical and mental health, and household characteristics26. Utilisation data are collected via frequency questions on categories of health, social and residential care in the preceding year, e.g., ‘how many times did you visit the GP in the last 12 months?’ While previous papers have estimated costs in TILDA27,28, these have been constrained by incomplete availability of unit costs in non-acute care and crude casemix estimates for acute care.
Combining the new unit cost database with TILDA offers the opportunity to estimate in the greatest detail yet health, social and residential care costs (henceforth, ‘health care costs’) among a population-representative sample of older people in Ireland. We supplement this new non-acute unit cost database with our own analyses of hospital inpatient admissions, adjusting for age, sex, diagnoses and discharge status, and a costing exercise of medications reported by TILDA respondents. The arising estimates can inform ongoing research studies, including those evaluating specific policies and models of delivery29, delineating patterns and trajectories of health care use30, surveying end-of-life needs31,32, and projecting future needs and costs33–35. They can also contribute to future studies both within TILDA, for example prediction exercises to identify high-cost users27; and in wider modelling frameworks, for example cost-effectiveness analyses that have to characterise disease trajectories and costs in different clinical populations36.
Our aims in this paper are to first document the methods by which health care costs are estimated in TILDA, and then to address the following research questions:
1. What are the health care costs for older people in Ireland? How are costs distributed across the population?
2. What is the underlying composition of these costs between primary and community care, hospital care, home care, residential care, and medications?
3. What individual-level predictors are associated health care costs?
This is a costing study using secondary data sources. Our main data source was TILDA, which recruited a population-representative sample of more than 8,000 community-dwelling people aged 50+ years at Wave 1 (2009–2011)37. Full details of the study design, recruitment, consent and data collection are available elsewhere26. Briefly, computer-assisted personal interview (CAPI) and a self-completion questionnaire (SCQ) are used to collect data on demographic and socioeconomic characteristics such as early life, household composition, employment history, income and asset levels, as well as detailed information on health status (e.g., diagnoses, functional status, self-reported physical and mental health) and healthcare utilisation. When a participant dies, a family member or close friend is approached to conduct a voluntary end-of-life interview (EOLI) on the decedent’s experiences in the last 12 months of life. This process, including the ethical guidelines and procedures, has been detailed elsewhere28. The EOLI represents a shortened version of the CAPI, asking the respondent questions on the decedent’s living situation, health, health care use and other factors.
The baseline sample were invited to participate in CAPI and SCQ follow-up at Wave 2 (2012), Wave 3 (2014), Wave 4 (2016), Wave 5 (2018) and Wave 6 (2021, delayed from 2020 by the COVID-19 pandemic). Ethical approval for each wave is obtained from the Faculty of Health Sciences Research Ethics Committee in Trinity College Dublin. Participants make an informed decision about their participation, receiving advanced notice and information booklets; they may refuse to take part in any study section or withdraw at any time without justification; for each CAPI and EOLI question, available answers include “Refuse to answer” and “Don’t know”.
Secondary data sources were two unit cost databases for non-acute costs24,38, and the Hospital Inpatient Enquiry (HIPE) database of admissions to public acute hospitals39. We also draw on Census data from the Central Statistics Office (CSO), for the purposes of population weighting, and the General Register Office (GRO) to identify deaths40. All deaths in the Republic of Ireland must be recorded with the GRO, and TILDA is linked to the GRO in a process described previously41.
In terms of perspective, we estimate the cost associated with providing the formal health care that TILDA participants and EOLI respondents report. We do not analyse or report out-of-pocket spending, which has implications for how costs are distributed between the system and households, and we do not analyse or report unpaid care provided by family and friends.
Dependent variable
The primary dependent variable is formal health care costs, which combines the estimated costs associated with acute and non-acute care, and medications, reported by participants.
For acute and non-acute care, TILDA collects data on the frequency (f) of use for a number (n) of categories (h), where n varies slightly between CAPI and EOLI, because hospice inpatient stays are not asked in the CAPI (implicitly assumed as zero). A unit cost (c) was identified for each category. Therefore, for each individual CAPI or EOLI (i), a specific acute or non-acute category (h) has associated costs (yi,h) given by:
Unit costs for non-acute care have been calculated in two prior costing exercises by Brick et al.38 and Smith et al.24 Hospital emergency department and outpatient unit costs have been calculated previously by Keegan et al.34 We were not aware of any unit costing exercise for acute inpatient admissions that was coherently linkable with individual TILDA data, and so we calculated acute inpatient unit costs using HIPE data in a procedure detailed in ‘Appendix 1’, which can be found as Extended data42. Briefly, in TILDA, we categorised each CAPI and/or EOLI to a category based on age, sex and diagnostic profile. In HIPE, we calculated the reimbursement due for each overnight adult admission to a public acute hospital in Ireland between 2009 and 2019 using the Healthcare Pricing Office (HPO) activity-based funding (ABF) guidance43, which determines reimbursement rates according to primary diagnosis and length of stay. We categorised each of these admissions by age, sex and diagnostic profile, and then calculated acute unit costs for each age/sex/diagnostic group as the mean reimbursement for an overnight stay in that group. We linked these acute unit costs to each CAPI and EOLI by age/sex/diagnostic profile, incorporating for EOLIs the additional cost associated with a death in hospital.
In all cases we chose the most recently available unit costs available. These most recent unit costs were calculated in different years. We standardised all unit costs to 2022, the year that the analyses were conducted, using the Consumer Price Index (CPI) for health44,45. In data processing we created sub-groups for ease of interpretation: primary and community care, hospital care, home care, residential care, and medications. Each category of care, its’ variable name in the most recent publicly available CAPI and EOLI, the unit cost source, the unit cost after adjusting to 2022 prices, and the sub-group to which it was allocated are presented in Table 1.
| Sub-Group | Category | Unit cost source | Unit cost (2022 EUR) | CAPI48 | EOLI§ |
|---|---|---|---|---|---|
| Hospital | Emergency department | Keegan et al.34 (i) | EUR 321 per visit | hu007 | xt_hu010 |
| Outpatient visit | Keegan et al.34 (i) | EUR 184 per visit | hu008 | xt_hu011 | |
| Overnight inpatient admits | Authors’ own(ii) | By age/sex/dx | hu010 | xt_hu013(iii) xt_cs021(iv) | |
| Primary and community | General Practitioner | Smith et al.24 (v) | EUR 49 per visit | hu005 | xt_hu005 |
| Public Health Nurse | Smith et al.24 (v) | EUR 60 per visit | hu015_01(vi) | xt_hu029_01 | |
| Occupational therapist | Smith et al.24 (v) | EUR 69 per visit | hu015_02(vi) | xt_hu029_02 | |
| Chiropodist | Smith et al.24 (v) | EUR 69 per visit | hu015_03(vi) | xt_hu029_03 | |
| Physiotherapist | Smith et al.24 (v) | EUR 69 per visit | hu015_04(vi) | xt_hu029_04 | |
| Speech & lang. therapist | Smith et al.24 (v) | EUR 69 per visit | hu015_05(vi) | xt_hu029_05 | |
| Social worker | Smith et al.24 (v) | EUR 47 per visit | hu015_06(vi) | xt_hu029_06 | |
| Psychologist | Smith et al.24 (v) | EUR 106 per visit | hu015_07(vi) | xt_hu029_07 | |
| Day care | Brick et al.38 (vii) | EUR 48 per visit | hu015_11(vi) | xt_hu029_08 | |
| Dentist | Smith et al.24 (v) | EUR 35 per visit | hu015_13(vi) | xt_hu029_10 | |
| Dietician | Smith et al.24 (v) | EUR 69 per visit | hu015_15(vi) | xt_hu029_12 | |
| Home | Home help(xiii) | Smith et al.24 (v) | EUR 35 per hour | hu015A | xt_hu022 |
| Personal care attendant(ix) | Smith et al.24 (v) | EUR 36 per hour | hu015B | xt_hu025 | |
| Meals on wheels | Brick et al.38 (vii) | EUR 12 per visit | hu015C | xt_hu027 | |
| Home care package(ix) | Smith et al.24 (v) | EUR 36 per hour | hu015D | xt_hu074 | |
| Residential | Nursing home(x) | Smith et al.24 (v) | EUR 1,722 per week | hu032 | xt_cs025 |
| Hospice | Brick et al.38 (vii) | EUR 999 per night | n/a(xi) | xt_cs023(iv) |
§ EOLIs are not published on the study website (tilda.tcd.ie), but access may be applied for at that location. (i) Keegan et al., estimated costs for 2018; per the CSO CPI Health, the multiplier from December 2018 to December 2022 was 1.076. (ii) Overnight admissions were costed using HIPE, detailed ‘Appendix 1’ in Extended data42. (iii) Where EOLI reports death in hospital, the unit cost for that admission is adjusted (see Appendix 1)42. (iv) Where EOLI reports people admitted to hospital or hospice as an inpatient, these episodes were costed using the relevant category unit cost and reported under that sub-group; where EOLI reports a decedent was living in a hospital or hospice as their main residence, these episodes were costed using the nursing home unit cost and reported under the sub-group ‘residential care’. (v) Smith et al., estimated costs for 2019; per the CSO CPI Health, the multiplier from December 2019 to December 2022 was 1.066. Smith et al., report different scenarios, we use the baseline public system unit cost in all cases. (vi) In the CAPI at Wave 1 and 2, these frequencies were binary (i.e., do you use this service?); for non-users we set yi,h=0; for those using the service we set yi,h to equal the age- and sex-adjusted median among service users in Waves 3-5. (vii) Brick et al., estimated costs for 2011; per the CSO CPI Health, the multiplier from December 2011 to December 2022 was 1.122. (viii) ‘Home help’ in TILDA is termed ‘Health Care Support Assistant’ in Smith et al. (ix) For ‘Home care package’ and ‘Personal care attendant’ in TILDA, we used ‘Health Care Support Assistant’ in Smith et al. (x) For ‘Nursing home’ in TILDA, we used ‘Long-term residential care’ in Smith et al. (xi) Hospice use is not part of the CAPI. CAPI, computer-assisted personal interview; EOLI, end-of-life interview; CSO, Central Statistics Office; CPI, Consumer Price Index; HIPE, Hospital Inpatient Enquiry; TILDA, The Irish Longitudinal Study on Ageing.
For medications, CAPI respondents detail the medications that they take “on a regular basis”, which includes prescribed medications, as well as those purchased over-the-counter, vitamins and supplements, and herbal products. Medication names are recorded as they are reported (either branded/generic product name or drug name), however strength and dosage are not captured. Each medication is assigned a WHO Anatomical Therapeutic Chemical (ATC) code where available relating to the drug they contain. We excluded reported products that do not have an ATC code, and any non-prescription items not reimbursed on Ireland’s community drug schemes (i.e., certain vitamins and over-the-counter products). For each included medication (m) we identified the associated cost (c), assuming the respondent was prescribed the WHO Defined Daily Dosage corresponding to the ATC code for one year, in the 2020 Health Service Executive reimbursement list46. Therefore, for each individual CAPI (i), reported regular usage of n medications has associated annual costs (yi,m) given by:
The EOLI does not collect medications data, but age- and sex-adjusted mean costs in the last year of life have been calculated previously47. We imputed into EOLIs yi,m using this mean by age and sex, after adjusting to 2022 using the CPI for health.
The primary outcome, an individual CAPI or EOLI’s total formal health care costs (Yi), expressed in euro (€, EUR) adjusted to 2022, is then calculated by summing yi,h for n categories of acute and non-acute care and adding the medications costs:
This outcome variable does not include some CAPI formal health care use data that might be considered relevant to health care costs. These variables, and the rationale for not including in this paper, are summarised in Table 2.
| Category | CAPI48 | Reason for exclusion |
|---|---|---|
| Optician | hu015_12 | No unit cost reported in Brick et al., Smith et al., or PSSRU 201949 |
| Hearing | hu015_14 | |
| Respite care | hu015_16 | |
| Consultant | hu062 | Binary, no frequency data to calculate costs |
| Operations | hu011 | No operation-specific data on which to base unit costs. |
| Public or private hospital? | hu014 | No unit costs available for private hospitals. |
| Private home care | hu076 | Not collected for all CAPI waves, and/or in all EOLI waves; excluded for consistency. |
| Private allied health and social care | hu084 |
We did not identify any health care use variables in the EOLI that are not in either Table 1 or Table 2. Those interested may check the full suite of TILDA variables at any time via the study website.
Independent variables
In multivariate regressions for our third research question, we identified predictors on a hypothesis-driven basis using the Andersen model of health care utilisation, which categorises potential predictors as predisposing, enabling, need or prior use50. Additionally we controlled for proximity-to-death effects using death dates for both CAPI and EOLI observations. The variables employed in multivariate regressions are summarised in Table 3.
| Group | Variable | Categorisation |
|---|---|---|
| Predisposing | Age§ | Years |
| Sex§ | Male | Female | |
| Enabling | Education: Highest achieved§ | Primary | Secondary | Tertiary |
| Medical card or GP card?*# | Yes, either/or | |
| Private insurance?*Ɛ | Yes, either as policy holder or on another’s policy | |
| Marital status | Married | Living with a partner ==1 Single | Widowed | Divorced | Separated ==0 | |
| Local region* | Dublin city and county | Urban area, not Dublin | Rural area | |
| Need | Cancer¥ | Has a doctor ever told you that you have ___? |
| Heart disease¥ | Has a doctor ever told you that you have ___? | |
| Multimorbidity¥ | Has a doctor ever told you that you have 2+ of the following: cancer, heart disease, kidney disease, liver disease, lung disease, Alzheimer’s disease and related dementias, hypertension; diabetes; stroke; arthritis; psychological issues including anxiety and depression; alcohol and/or drug abuse? | |
| Instrumental Activities of Daily Living (IADLs)*51 | Because of a health or memory problem, do you have difficulty doing any of the following activities: preparing a hot meal, shopping for groceries, making telephone calls, taking medications, managing money, doing household chores? Total difficulties (/6)= 0 | 1 | 2+ | |
| Physical exerciseβ | Do you engage in vigorous physical exercise at least weekly? | |
| Prior use | Emergency department (ED) admissions+ | How many ED admissions in the prior interview? Total admissions = 0 | 1 | 2+ |
| Proximity to death | Last two years of life (L2YOL)θ | Among CAPI sample, did the participant die within two years of the interview? |
| Last year of life (LYOL)α | Was the participant in the last year of life (i.e., is this a CAPI observation or an EOLI observation)? |
§ For both CAPI and EOLI observations, these variables are taken from the baseline enrolment data (and EOLI age adjusted to age at death using date of death). # Medical cards are provided on a means-tested basis, and provide free access to inpatient and outpatient public hospital care, to GPs, and to prescription drugs; GP cards are means-tested under the age of 70 years and provided universally thereafter, and afford free access to the GP.52 Ɛ Private insurance is voluntary in Ireland; it provides access to some additional facilities and expedites access to certain services. * For CAPI observations, these variables are taken from the Wave 5 responses: for EOLI observations, these variables are taken from the EOLI or if the respondent did not know or refused to answer they are taken from the last CAPI prior to death. ¥ Diagnoses are treated as absorbing states; for CAPI observations, a reported diagnosis at any Wave up to and including Wave 5; for EOLI observations, a reported diagnosis at any CAPI or in the EOLI. β For CAPI observations, these variables are taken from Wave 5; for EOLI observations, these variables are taken from the last CAPI prior to death. + For CAPI observations, prior use variables are taken from Wave 4; for EOLI observations, these variables are taken from the last CAPI prior to death. θ Identified via GRO linkage; included in CAPI and pooled regressions only; always ==0 in EOLI sample. α Included in pooled regression only, has a fixed value within CAPI (==0) and EOLI (==1) samples. CAPI, computer-assisted personal interview; EOLI, end-of-life interview; GRO, General Register Office.
In the main paper we focus on two sets of interviews: CAPIs at Wave 5, and EOLIs at any wave. We choose Wave 5 as the most recent conducted prior to the COVID-19 pandemic; Wave 6 interviews were conducted during 2021, which was an atypical period of health care utilisation and is likely not generalizable to other years. By Wave 5 (2018), the baseline sample (aged 50+ years in 2009–2011) are nearly all aged 55+ years (the only exceptions are those who enrolled aged <50 years old while participating as the spouse of a participant aged >50 years old). Therefore, we excluded those aged <55 years from all analyses; the numbers are presented with the population-level weights in ‘Appendix 2’, found as Extended data42.
We include EOLIs from all waves prior to March 2020, since wave-by-wave samples are relatively small, these observations heavily influence cost estimates (see Results for full details), and we consider it a reasonable assumption that pre-pandemic deaths in all TILDA years are substantively comparable. The sample eligibility was therefore defined as all Wave 5 CAPI participants aged 55+ years, and all EOLIs aged 55+ years at any wave, except (i) deaths occurring after 29/2/20, and (ii) the deaths for participants at Wave 5 and so individuals already in our sample. We summarise how this sample is reached in the Results and present the characteristics of those excluded in ‘Appendix 2’42.
As such our reported estimates reflect our best understanding of health care costs among older people in Ireland in 2019, updated for inflation to 2022.
Prior studies have found that missing data in both CAPIs and EOLIs is relatively rare; e.g., at baseline this was less than 1% for predisposing, enabling and need characteristics (Table 3 in May et al., 202233), although there have been small increases in such missingness wave-on-wave. For the dependent variable, prior analyses of TILDA have suggested that of all categories in Table 1, four account for over 80% of total costs in the CAPI: GP, inpatient, outpatient and home help27,28. Any sample-eligible CAPI or EOLI that was missing two or more of these four categories was flagged and removed from primary analysis as having insufficient outcome data. For those interviews missing one or fewer of these categories, and or missing any other categories of health care frequency, we imputed age- and sex-adjusted medians. For independent variables, any sample-eligible CAPI or EOLI that was missing three or more baseline predictors was removed from primary analysis as having insufficient baseline data. For those interviews missing two or fewer baseline variables, we imputed the same individual’s data from the most recently available prior wave.
TILDA in Wave 1 aimed to recruit a population-representative sample of community-dwelling adults aged 50+ years but the sample inevitably differs from the population, and this variation will have increased if those who die or drop out or have missing data differ systematically from those who continue to take part. We addressed this sampling uncertainty, and the concomitant risk of bias, through sampling weights that used the CSO population data to calculate the probability of any given participant having been included in the sample. For this paper we weighted by age (five-year bands), sex (male or female), and last year of life (=1 for EOLIs, 0 for CAPIs). Weights were calculated using the CSO population data for 2019, the most recent pre-pandemic year full data were available. See ‘Appendix 2’42.
All analyses were performed in Stata version 15 (RRID:SCR_012763)53; an open access alternative that can perform equivalent tasks is R (RRID:SCR_001905)54. For research questions 1 and 2, we report descriptive and distributional statistics after applying the population weights. For research question 3, we run multivariate regressions in the eligible Wave 5 CAPIs, in the eligible EOLIs, and in the CAPIs and the EOLIs pooled. In all cases we modelled outcomes using a generalised linear model with a power link, selected using information criteria before inspecting or interpreting results55. Prior to estimating results we assessed collinearity of predictors using the –collin- command. For each association between predictor and outcome, we report dy/dx using the –margins- command; this reflects the estimated mean association with outcome of increasing the value of the predictor by one point while holding all other values in the model constant.
Additional data and sensitivity analyses
For reader information, we present the following data before and after weighting in supplementary materials:
We performed sensitivity analyses on our regressions, presented in Appendices:
Diagnostic checks for model choice and collinearity are presented in ‘Appendix 7’42. Additional information on the medications costing exercise are presented in ‘Appendix 8’42.
Figure 1 details how the analytic samples were reached. There were 5,222 completed CAPI interviews at Wave 5, of which 96 were aged <55 years, one had insufficient baseline data, and 20 had insufficient outcome data. This gave a CAPI analytic sample of 5,105 Wave 5 participants. There were 892 completed EOLI interviews at time of data analysis (Q3 2022), of which seven were for people aged <55 years, three concerned deaths occurring March 2020 onwards, 11 had insufficient outcome data, and 108 were already in the CAPI sample. This gave an EOLI analytic sample of 763 deceased participants. When the CAPI and EOLI data were pooled, this was an analytic sample of 5,868 unique individuals (Figure 1).
The analytic samples are provided in Table 4, after weighting. The 5,105 CAPI observations were equivalent to 1,207,660 people at the population level, and the 763 EOLI observations were equivalent to 28,466. Combined this was 1,236,126 people aged 55+ years in Ireland in 2019.
NTILDA is the number of observations in each sample (CAPI/EOLI/ALL). Npop is the number of people in each sample (CAPI/EOLI/ALL) at the population level after weighting; npop is the number of people in each cell at the population level after weighting. For unweighted samples and TILDA cell sizes, see ‘Appendix 2’ in Extended data42. For definitions of variables, see Table 3. CAPI, computer-assisted personal interview; EOLI, end-of-life interview; TILDA, The Irish Longitudinal Study on Ageing; IADL, Instrumental Activities of Daily Living; ED, Emergency department; L2YOL, last two years of life; LYOL, last year of life.
There were large differences between the EOLI (last year of life) and CAPI (not last year of life) samples on all baseline predictors except region. More than two thirds of those in the last year of life were aged over 75 years, and more than three quarters not in the last year of life were aged under 75 years. Males were slightly more represented among the EOLI than CAPI samples, reflecting higher male mortality rates. The younger CAPI interviewees had higher average educational achievement, reflecting cohort effects in access, and higher prevalence of marriage, reflecting rising marriage rates in the middle of the 20th century and lower widowhood effects. Those in the last year of life were much more likely to have a medical card, reflecting wider entitlement from the age of 70 years, but less likely to have private insurance. EOLI observations had much higher prevalence of serious illness, functional impairment, and ED attendance; but much lower prevalence of regular physical exercise. In the pooled sample, patterns of characteristics substantively reflect the CAPI sample as these are 87% of observations before weighting and 98% after weighting.
What are the health care costs for older people in Ireland? How are costs distributed across the population?
Total formal care costs, in the CAPI and EOLI samples and pooled together, are presented in Table 5. Mean costs in the weighted sample were EUR 8,053, comprising EUR 6,624 in the CAPI sample and EUR 68,654 in the EOLI. Typical for cost data, there is considerable right-hand skew in all samples.
The distribution of formal costs across deciles are presented in Figure 2, after weighting. The skew is again heavily evidenced here: over 70% of people have costs less than EUR 2,000 a year, and the top 10% of people have mean costs of EUR 59,654.
The corresponding population-level costs are presented in Table 6. Total estimated population-level costs are EUR 9,954,054,582. Almost three quarters (74%) of these costs are accounted for by the 10th decile and another 16% by the ninth decile. Therefore, an estimated 90% of health care costs among people aged 55+ years are accounted for by 20% of users.
For equivalent data in the CAPI and EOLI samples separately, see Appendix 342.
What is the underlying composition of these costs between primary and community care, hospital care, home care, residential care, and medications?
The composition of costs for CAPI, EOLI and pooled samples are presented in Figures 3a, 3b and 3c, respectively. Hospital costs accounted for the majority of the dependent variable in all three cases; the most visible difference between CAPI and EOLI costs were those for residential care, which accounted for a far higher overall proportion among those in the last year of life.
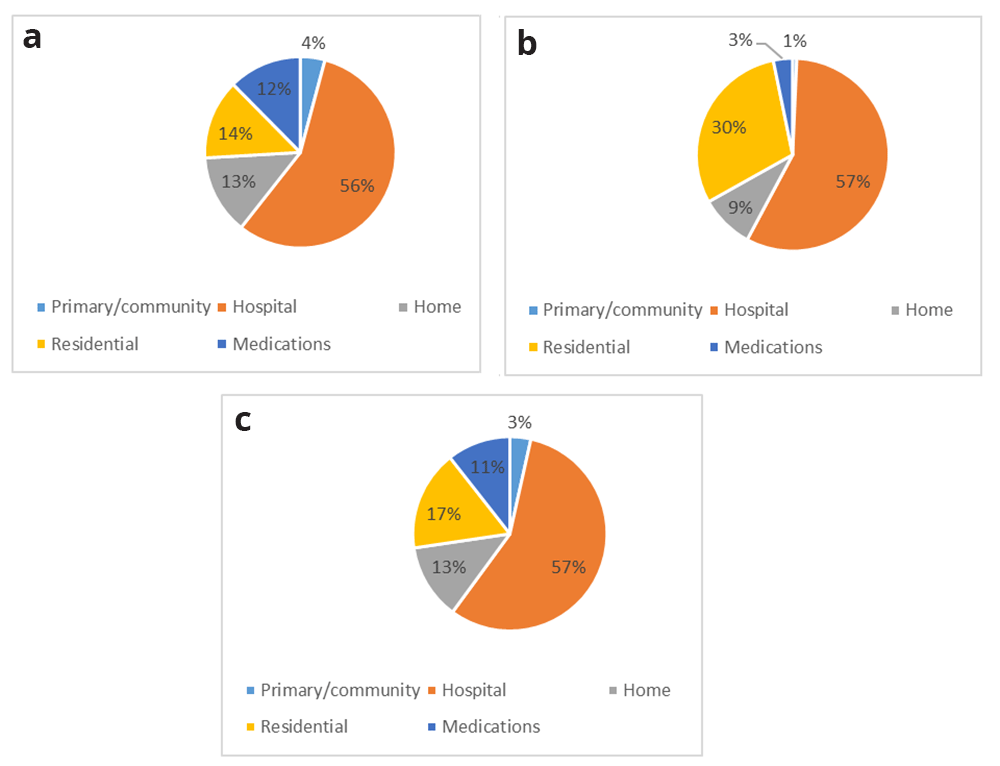
(a) CAPI, (b) EOLI and (c) pooled (ALL) sample. CAPI, computer-assisted personal interview; EOLI, end-of-life interview.
The composition of costs across deciles of total health care costs in the pooled sample is presented in Figure 4. The substantive jump between the eighth and ninth decile, already highlighted in Table 6, is mainly explained by hospital costs. The even larger jump between the ninth and 10th decile is explained by large increases in hospital, home and residential care costs.
The composition of costs among non-zero users, across 20 quantiles of total health care costs in the pooled sample, is presented in Figure 5. Among lower cost users, primary care and pharmacy costs dominate. From the second to 17th quantile, hospital costs account for a consistently increasing proportion. Home care costs are close to zero until the top five quantiles, thereafter, accounting for 2–12%. Residential care costs are close to zero until the top two quantiles, accounting for over a quarter of costs in the highest-cost group.
For equivalent data in the CAPI and EOLI samples separately, see Appendix 442.
What individual-level predictors are associated health care costs?
The results of the multivariate regressions are presented in Table 7. Statistically significant results are highlighted in bold.
For variable definitions, including reference cases, see Table 3. For full regression output including p-values, see Appendix 6 in Extended data42. dy/dx: the marginal effect; the estimated mean association with outcome of increasing the value of the predictor by one point while holding all other values in the model constant. CI, confidence interval; CAPI, computer-assisted personal interview; EOLI, end-of-life interview; IADL, Instrumental Activities of Daily Living; ED, Emergency department; L2YOL, last two years of life; LYOL, last year of life.
In the CAPI sample, the largest associations were 2+ IADLs, which was associated with EUR 21,376 higher costs compared to none (95% confidence interval: 11,714 to 31,037) and being in the last two years of life (+ EUR 16,250; 7,304 to 25,195). Other variables positively associated with outcome were: older age; medical card entitlement; living in a town or city other than Dublin; diagnoses of cancer, heart disease or multimorbidity; and prior ED attendance. Negative associations were college education and engaging in regular physical exercise, which in this context is a proxy for unobserved general health. In the EOLI sample, the largest association was again 2+ IADLs (+ EUR 30,637; 20,287 to 40,987). Other positive associations were cancer diagnosis and multimorbidity. Negative associations were male sex, living outside Dublin, regular exercise and multiple prior ED attendances.
In the pooled sample, results were substantively consistent with the CAPI, reflecting the fact that these are 98% of observations in the weighted sample. The largest associations were again multiple IADLs (+ EUR 21,437; 12,763 to 30,112), and proximity to death variables in the last two years of life (+ EUR 17,325; 8,439 to 26,210) and in the last year of life (+ EUR 17,865; 9,875 to 25,855).
This paper presents the most comprehensive picture to date on individual-level health care costs for older people in Ireland. We found that, adjusted to end 2022, mean costs among people aged 55+ years were EUR 8,053, with a large differential between those in the last year of life (EUR 68,654), and not in the last year of life (EUR 6,624). Hospital costs accounted for over half of costs in all three sampling frames. There was a very large skew in the data: the top 20% of users accounted for 90% of all costs at the population level, and the top 10% accounted for 74%. In multivariate regressions, multiple IADLs and proximity to death had the largest associations with outcome. In sensitivity analyses, our results are substantively similar for the IADL and proximity to death associations, and >95% of all associations in Table 7 have the same interpretation in these sensitivity analyses (Appendix 6)42.
The large association between multiple IADLs and costs is not surprising, and in part reflects the high level of health care worker time that are required to support this population. Nevertheless, the magnitude of association, and in particular that this variable is more strongly predictive of costs than proximity to death, is somewhat unexpected and has important policy implications. As the population ages, and in particular as dementia becomes more prevalent, the number of people living with multiple IADLs will grow33,56–58. Optimising care and supports for this group, in particular to support ageing in place and to minimise avoidable acute hospital admissions and residential care costs, remains an urgent priority29–32.
The significant differential between those in the last two years of life and not approaching end of life reflects a long-standing evidence base that proximity to death is a key determinant of health care costs. There were 31,184 deaths recorded in Ireland in 2019, of which over 28,000 (91%) occurred in people aged over 55 years59. The population-level costs of end-of-life care are therefore approximately EUR 2 billion annually. Total health spending in Ireland in 2019 was EUR 25.3 billion60,61. This suggests that the <1% of people who die each year in Ireland account for around 8% of health care spending, although the true ratio is sensitive to recent rapid increases in both inflation and public health spending, and the fact that our estimates likely undervalue total spend by using public service unit costs only. This ratio is consistent with what has been reported in other high-income countries62. The large projected growth in the number of older people dying annually as the population ages emphasises the urgent need to plan and fund palliative and end-of-life care services.
Some of the other associations were predictable in the context of prior literature. Older age is associated with somewhat higher costs compared to younger people (though this is heavily tempered by proximity-to-death dynamics, and more observable for social care than health care)5. Men have lower costs in the last-year-of-life cohort due to the higher prevalence of sudden death, but there is no apparent association in the whole population. Socioeconomic disparities in health are reflected in lower costs among those who stayed longer in education63. Medical card entitlements lead to higher health care use in the general population64,65, but in the end-of-life cohort where entitlements are more universal, they have no relationship. This relationship between entitlements and costs is complicated by potential confounding by socioeconomic and health status; those entitled to a medical card are more likely to have care needs that are not captured in the model. Diagnoses of serious disease necessitate higher health care costs66–68 and regular physical exercise protects against episodes of ill-health69,70, although in our data this is likely not causal but reflects the better health of regular exercisers. Prior patterns of hospital admittance strongly predict costs71. One surprising result was the association between region and outcome: those living outside Dublin in other urban settings had higher costs in the general population, but both urban and rural dwellers had substantially lower costs than those in the capital in the end-of-life cohort. Complex patterns of use by geographical region are commonplace among older populations72,73, and this warrants further investigation to pick apart issues of need, access and value74.
While skew in health care costs is a long-established phenomenon, the distribution in our data is still unusual. The historic interest has been in an ‘80–20 ratio’, where 20% of people account for 80% of spending, but we find that 20% of people account for 90%. Taking into account that our calculations are in the population aged 55+ years, and older people account disproportionately for health care spending, the ratio at the population level must be still more imbalanced. Ex ante identification of people who account heavily for health care costs, identifying and addressing low-value care, and reforming provision for an age of multimorbidity and ongoing supportive care are all strategies with enormous potential fiscal pay-offs, as well as improved outcomes for patients.
The estimation that hospital costs account for nearly 60% of total costs is higher than comparable data in England (50%)75. This is potentially associated with Ireland’s historic reliance on acute care and weak primary care capacity, and implies opportunity to reduce hospital costs through more cost-effective models of community care delivery16,21. Such aims are consistent with the ongoing Sláintecare reforms.
TILDA collects all CAPI data by self-report, and all EOLI by interview with a family member or friend, which may result in omissions and recall bias among both predictors and outcome. Absent a unique identifier in routine data, TILDA is nevertheless among the most powerful sources of individual-level data for understanding health care costs among older people in Ireland.
Our unit cost estimates do not take account of differential costs in private settings, which by volume account for approximately 20% of care in Ireland’s mixed system18,65. This means that our total reported costs are likely underestimates, and that interpreting associations between outcome and variables strongly associated with use of private care (e.g., health insurance, socioeconomic status) must be done with caution. TILDA collects frequency data on private hospital and home care use, meaning that future work can address this gap if credible unit costs of private care can be identified. Proportion of use that is private among different allied and social care categories varies widely, and as such so does the public/private distinction. We are unable to quantify the distribution of costs between payers (public and private care, and out-of-pocket costs). Many hospital engagements in Ireland are ‘day cases’; engagements not requiring an overnight stay. While TILDA captures ED attendances and same-day discharge following emergency admissions are included in our reported costs, we don’t include diagnosis-specific costs for outpatient engagements or procedures but instead estimate resources at a flat unit cost rate. Healthcare Pricing Office costs for inpatient stays do not include superannuation, while unit costs for other types of care do.
Hospital costs account for a majority of overall costs, but unit costs for acute care are age/sex/diagnosis-adjusted national averages only. While we have adjusted for discharge status (alive/dead; see Appendix 1)42, and age/sex/diagnostic profiles capture a significant proportion of proximity to death among those discharged alive, hospital costs are still highly heterogeneous within each age/sex/diagnostic category, reflecting myriad factors including physician and patient preferences, access, specific hospital setting, and discharge location options. Future work might address this to some extent; e.g., HIPE records whether a person was discharged home or to a hospice or a nursing home. However, arising estimated costs would be contingent on additional unverifiable assumptions and so come with increased risk of new biases. The promised implementation of a unique identifier would provide ‘true’ individual-level costs in HIPE against which different cost mix methods in TILDA could then be benchmarked.
The COVID-19 pandemic complicated choice of an appropriate timeframe. For CAPI observations, we used Wave 5 as the most recent pre-pandemic wave (2018), and for EOLI observations, we used all pre-pandemic observations to maximise sample size in a group that has disproportionate influence on estimates. Our reported estimates reflect best understanding of health care costs among older people in Ireland in 2019, updated for inflation to 2022. The information provided in this manuscript and in the appendices equips readers to revise group averages using the CPI for health, and/or to weight at the population level for other years using CSO data, should they choose to do so. Parsing the effects of COVID-19 on general health care use, both during the heights of the pandemic 2020–2021, and into the future, have been examined to some extent in other TILDA analyses and are an important topic for ongoing study.
TILDA recruited a population-representative sample in 2009–2011, but attrition to Wave 5 (2018) may have undermined this representativeness. We weighted using age, sex and last year of life since these data are theoretically associated with outcome and easily available from the CSO. Prior weighting exercises in the TILDA CAPI have also incorporated education, marital status and geographical location to maximise generalisability76. While these data are available via the census for the CAPI, the last matching exercise by the CSO to the GRO was after the 2016 Census. Our strategy therefore reflects the best approach with publicly available data for all CAPI and EOLI observations. Future work may seek to improve the precision of weighting, for example by getting additional ‘enabling’ variables on decedents from the CSO’s data controllers.
High-quality data on health care costs are essential to support monitoring, planning and evaluation of services, and the allocation of scarce resources to maximise public welfare. By combining newly available unit cost data in non-acute care, our own estimates of acute costs, and the rich data in TILDA, we present the most comprehensive picture to date on individual-level costs among older people in Ireland. We quantify more precisely some well-known relationships, particularly the high costs associated with end-of-life care, and also identify some potentially underestimated dynamics, in particular that multiple functional impairments appear a more significant driver of costs than age, diagnosis, multimorbidity or proximity to death. The derived estimates can inform multiple ongoing research studies and policy activities, as well as providing a foundation for future work, which should include consideration of private provider costs in Ireland’s unusual mixed system.
Ethical approval for each wave of the TILDA study is obtained from the Faculty of Health Sciences Research Ethics Committee in Trinity College Dublin. Participants are provided with sufficient information to make an informed decision about their participation including advance notice of the study. Written consent is obtained for separate components of the study (i.e., interview, health assessment, blood samples); participants may refuse to take part in or withdraw at any time without justification. Ethical approval for the secondary analysis of TILDA data used in this study was part of this overall approval.
Researchers interested in using regular waves of TILDA data may access the data for free from the following sites: Irish Social Science Data Archive (ISSDA) at University College Dublin (http://www.ucd.ie/issda/data/tilda/); Interuniversity Consortium for Political and Social Research (ICPSR) at the University of Michigan (http://www.icpsr.umich.edu/icpsrweb/NACDA/studies/34315).
Replication of the results reported in this article requires access to the full TILDA dataset, which is held on secure servers at the study site at Trinity College Dublin (TCD). Researchers seeking access to the full TILDA dataset may apply to access the data on the TCD campus (tilda.tcd.ie); applications are considered on a case-by-case basis; all Stata do files and code employed in this paper will be made available to applicants on request.
Open Science Framework: Appendices to 'Formal health care costs among older people in Ireland: methods and estimates using The Irish Longitudinal Study on Ageing (TILDA)'. https://doi.org/10.17605/OSF.IO/76SYK42.
This project contains the following extended data:
- Appendix 1.docx (information on calculations of costs associated with inpatient hospital admissions in TILDA)
- Appendix 2.xlsx (characteristics of the sample, and those excluded per Figure 1)
- Appendix 3.docx (calculations of health care costs for older people in Ireland and distribution across the population)
- Appendix 4.docx (calculations of underlying composition of health care costs between primary and community care, hospital care, home care, residential care, and medications)
- Appendix 5.docx (summary statistics and distributions for total health care costs in CAPI waves 1–4)
- Appendix 6.docx (sensitivity analysis)
- Appendix 7.docx (diagnostic checks for model choice and collinearity)
- Appendix 8.docx (information on the medications costing exercise)
- STROBE checklist
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Is the rationale for developing the new method (or application) clearly explained?
Yes
Is the description of the method technically sound?
Yes
Are sufficient details provided to allow replication of the method development and its use by others?
Yes
If any results are presented, are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions about the method and its performance adequately supported by the findings presented in the article?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Health economics
Is the rationale for developing the new method (or application) clearly explained?
Yes
Is the description of the method technically sound?
Yes
Are sufficient details provided to allow replication of the method development and its use by others?
Yes
If any results are presented, are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions about the method and its performance adequately supported by the findings presented in the article?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Dementia care, health economics, health services research
Is the rationale for developing the new method (or application) clearly explained?
Yes
Is the description of the method technically sound?
Yes
Are sufficient details provided to allow replication of the method development and its use by others?
Yes
If any results are presented, are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions about the method and its performance adequately supported by the findings presented in the article?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: health economics
Is the rationale for developing the new method (or application) clearly explained?
Yes
Is the description of the method technically sound?
Yes
Are sufficient details provided to allow replication of the method development and its use by others?
Partly
If any results are presented, are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions about the method and its performance adequately supported by the findings presented in the article?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Health Economics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 1 04 Mar 23 |
read | read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with HRB Open Research
Already registered? Sign in
Submission to HRB Open Research is open to all HRB grantholders or people working on a HRB-funded/co-funded grant on or since 1 January 2017. Sign up for information about developments, publishing and publications from HRB Open Research.
We'll keep you updated on any major new updates to HRB Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)