Keywords
ANCA-associated vasculitis, registry, outcomes, death, end-stage-kidney-disease, urine soluble CD163 (usCD163)
This article is included in the Dementia Trials Ireland (DTI) and Dementia Research Network Ireland (DRNI) gateway.
ANCA-associated vasculitis, registry, outcomes, death, end-stage-kidney-disease, urine soluble CD163 (usCD163)
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a rare multisystem autoimmune disease, characterised by necrotising pauci-immune small-vessel vasculitis. It comprises three clinico-pathological syndromes: granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (EGPA). The estimated overall annual incidence of AAV is 13–20 cases/million1 with a prevalence of 300–421 cases per million2.
Significant treatment advances have occurred over the past 70 years with the advent of corticosteroid use in the 1950s, followed by the addition of cyclophosphamide in the 1960s, resulting in an improvement in two-year survival from 20 to 80%3. The introduction of ANCA testing in the 1980s improved detection of AAV and augmented disease awareness. With the achievement of remission in the majority, accompanied by an improvement in immediate survival, focus has now shifted to examining long-term outcomes, influenced by both treatment and the disease itself. AAV still carries a 2.7-fold increased risk of death compared to the general population4. Immunosuppression used to induce and maintain remission is a double-edged sword with >80% experiencing adverse events5. Given the relapsing and remitting nature of AAV, short randomised controlled trials (RCTs) are unable to provide robust evidence on longer-term outcomes, such as long-term treatment safety and efficacy in the biologic era. Additionally, RCTs do not provide information on diverse populations who may not consent to inclusion in trials, such as those with very severe disease admitted to ICU at presentation, older patients, marginalised populations and pregnant women. This knowledge gap has prompted the development of longitudinal vasculitis registries, including at least eight in Europe6. Increasingly, patient registries, linked to biobanks, are being used to facilitate longitudinal cohort studies in rare diseases, with a recent European effort to ensure standardisation and interoperability, via initiatives from FAIRVASC and the European Vasculitis Society (EUVAS) registries group6. This will allow collaborative research using aggregated data to analyse critical outcomes at a larger scale with sufficient power.
The Irish Rare Kidney Disease (RKD) registry and biobank was created in collaboration with the Irish national vasculitis patient organisation (https://vasculitis-ia.org/) in 2012 with the aim of collating clinical data and bio-samples from patients with vasculitis, and from disease and healthy controls on a national level. Importantly, this registry included, from inception, input from patient advocates (Vasculitis Ireland Awareness), recruitment during acute hospital admissions (allowing for pre-treatment data and sampling) as well as the involvement of a range of specialists including nephrology, rheumatology and immunology, allowing recruitment of broader clinical phenotypes. The current study arose from a need to appraise current practice and outcomes in the real-world setting. It aims to describe the long-term outcomes and potential prognostic factors at presentation in this large multicentre prospectively recruited cohort of AAV patients in Ireland.
(A) RKD registry. The Irish Rare Kidney Disease (RKD) registry and biobank was established in 2012, recruiting from eight clinical centres (Extended data: Supplementary Table 1)7. All patients >16 years of age with prevalent vasculitis were eligible for recruitment and diagnoses were assigned according to the 2012 Chapel Hill Consensus nomenclature8. Central ethical approval was granted by the Tallaght University Hospital/St. James’s Hospital Joint Research Ethics Committee (reference 2019-08 List 29 (07)) on 30th August 2019, and locally by each study site, and all participants provided written informed consent. Central storage of anonymised registry data is hosted on a secure password-protected web application (REDCap: https://www.project-redcap.org/)9,10.
(B) Prospective AAV cohort. In this paper we report in detail on participants with definite AAV defined by relevant clinical features of GPA, MPA or EGPA8, with either positive anti-myeloperoxidase (MPO) or anti-proteinase 3 (PR3) serology11 and/or diagnostic histopathology. Patients with secondary vasculitis or dual anti-glomerular basement membrane disease were excluded from this analysis12. Patients who were positive for both MPO- and PR3-ANCA (n = 5) were classified according to whichever titre was higher13. Analysis was restricted to only those diagnosed from 1st January 2012 onwards, to eliminate retrospective recruitment bias (Extended data: Supplementary Figure 1)7.
The following data were collected prospectively: demographics, date of diagnosis and symptom onset, vasculitis characteristics (diagnostic subtype, organ involvement, ANCA specificity), treatment, histopathology, radiology, complications and follow-up clinical encounters (including disease activity assessed by the Birmingham Vasculitis Activity Score version 3 (BVAS v3)14), urinalysis, pertinent laboratory data and exploratory biomarkers. Encounters were usually recorded at three-monthly clinic intervals and at the time of relapse, with a targeted annual minimum. Urine soluble CD163 (usCD163) was measured if clinically indicated in a central clinical laboratory using an accredited ELISA (Euroimmun, GMBH). The observation period was from the date of diagnosis to the occurrence of the event of interest (end-stage kidney disease (ESKD), death or last follow-up). Data collection is in agreement with the core dataset and interoperability principles outlined by EUVAS6.
The degree of certainty in the AAV diagnosis was estimated using a novel pragmatic diagnosis confidence matrix (Extended data: Supplementary Figure 2)7; only participants with a “definite” diagnosis were included in the prospective AAV cohort. ESKD was defined by a consensus EUVAS decision as the commencement of renal replacement therapy for at least 90 days (or death within 90 days), sustained estimated glomerular filtration rate <15ml/min and/or renal transplantation. The estimated glomerular filtration rate (eGFR, ml/min/1.73m2) was calculated using the CKD-EPI formula. The ‘combined burden of events’ (CBOE) score is a representative summary variable to describe cumulative adverse events based on the Common Terminology Criteria for Adverse Events (CTCAE) and sub-categorised into “infection”, “leucopenia” and “other”, each rated on a score of 1–4 based on severity5.
Primary outcomes were time to ESKD and time to death. A composite of time to ESKD or death and factors associated with the occurrence of adverse events were also investigated. Continuous variables are reported as mean (standard deviation, SD) or median (interquartile range, IQR, if not normally distributed), and compared using the independent sample t-test or Mann-Whitney U test, respectively. Categorical variables are summarised by frequency and percentage (%) and compared using the chi-square test. Imputation was performed, using the mice R package (Version 3.13.0)15, to estimate missing (9.1%) eGFR values16. Survival probability was determined using Kaplan-Meier survival analysis. Analyses were censored for death or last follow-up and considered, firstly, the events only within the first year and, secondly, the events over the entire period of observation. Between group comparisons were performed using the log-rank test. Uni- and multivariate Cox regression analyses were performed separately to investigate predictors of mortality, ESKD, and the composite of ESKD/death. A backward stepwise method, in conjunction with a priori knowledge of significant confounders, was used to identify the final independent risk factors in the adjusted models. Potential confounders included both patient- and disease- characteristics: age and eGFR at diagnosis, sex, AAV phenotype, ANCA serology, organ involvement and CBOE score (or its components). Diagnosis BVAS was excluded from models due to its degree of missingness (28.5%). “Renal involvement” as a binary variable was also excluded due to strong correlation with diagnosis eGFR. The ‘surv_cutpoint’ function of the survminer R package (Version 0.4.9)17 was used to identify the optimal binary cut-point for continuous predictors of importance, based on the maximally selected log-rank statistic18. Cox proportional hazard ratios (HR) with 95% confidence intervals (95% CI) are reported, after evaluating the weighted and scaled Schoenfeld residuals to ensure the proportional hazards assumption was met. We performed multivariate logistic regression to identify key predictors of a CBOE score >8. Gender, AAV phenotype and induction treatment were included in this analysis as fixed factors, while diagnosis age and eGFR were continuous covariates. A two-tailed P value <0.05 was considered statistically significant. All statistical analyses were performed using R (Version 4.0.4). All survival analyses were performed using the survival R package (Version 3.2-11)19.
(A) RKD registry. As of 31st May 2021, 842 unique patients with vasculitis were recruited (Extended data: Supplementary Table 2)7. Most were Caucasian (97.7%) with a median diagnosis age of 59 years (IQR 48–69) and a male: female ratio of 1.2: 1. The commonest diagnosis was AAV (82.3%). A total of 644 (76.5%) underwent diagnostic biopsy, of which 532 (63.2% of all cases) showed definitive vasculitis. According to a diagnosis confidence matrix (Extended data: Supplementary Figure 2)7, 750 (89.1%), 47 (5.6%) and 45 (5.3%) had a definite, possible and probable diagnosis confidence, respectively.
(B) Prospective AAV cohort. Of the 612 patients with a definite AAV diagnosis, 397 were prospectively recruited from 2012 onwards (Extended data: Supplementary Figure 1)7; median age at diagnosis was 64 years (IQR 55–73) and 230 (57.9%) were male. The most observed phenotype was MPA (58.9%) and most (341, 85.9%) had renal involvement. Median baseline eGFR was 28 ml/min/1.73m2 (IQR 13–62). Median duration from symptom onset to diagnosis was 9.4 weeks (IQR 4.3–25.6) and follow-up continued for a median of 33.5 months (IQR 10.7–52.7). Complete patient- and disease-characteristics, including induction and maintenance treatment, are detailed in Table 1 and Extended data: Supplementary Table 37.
Diagnosed with ‘definite’ AAV from 1st January 2012 onwards and recruited to the RKD registry before 31st May 2021. ‘Other’ Induction treatment includes AZA, MMF, MTX or GCC only. ‘Other’ Maintenance treatment includes: 1 <= of AZA, MMF, MTX, Tacrolimus, Ciclosporin, Cyclophosphamide, Mepolizumab, Ustekinumab, Hydroxychloroquine, Belimumab or unknown maintenance therapy. See Extended data: Supplementary Table 3 for further details on maintenance RTX group. Missing data: Induction treatment N = 6. AAV = ANCA associated vasculitis, eGFR = estimated Glomerular Filtration Rate (CKD-EPI), BVAS = Birmingham Vasculitis Activity Score, CYC = Cyclophosphamide, RTX = Rituximab, AZA = Azathioprine, MMF = Mycophenolate mofetil, GCC = Glucocorticoids. VINE = Vasculitis Ireland Network. IQR = interquartile range; SD = Standard deviation
In the first-year post diagnosis, twenty-two (5.5%) patients died, and actuarial survival was 93.8% (95% CI 91.3–96.3, Figure 1a). Infection was the leading cause of death (45.5%), followed by active vasculitis (18.2%, Extended data: Supplementary Table 47). Age, degree of renal impairment at diagnosis, weighted infection and leucopenia adverse event scores, and cardiovascular involvement were independent predictors of one-year mortality on multivariate analysis (Table 2). An eGFR cut-off of <20ml/min/1.73m2 identified those at highest risk of one-year mortality (Figure 1b)18. As the CBOE score rose, mortality increased. The optimal CBOE score (Extended data: Supplementary Figure 3)7 mortality cut-off was >8 (Extended data: Supplementary Figure 4)7, which was associated with a one-year survival probability of 55.9% (41.0–76.2) compared to 97.5% (95.8–99.2) for those with a score ≤8 (Figure 1c). Renal dysfunction severity was an independent risk factor for a CBOE score >8 (Table 3).
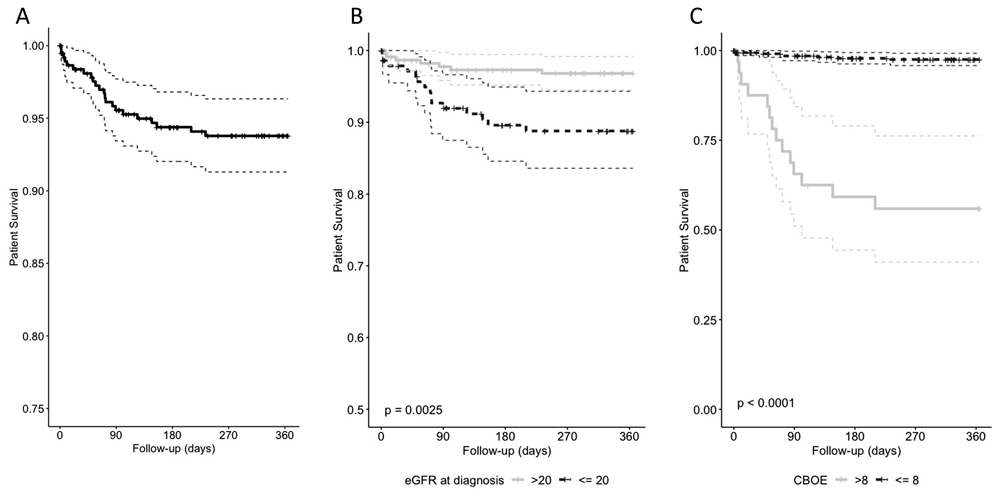
Optimal cut-points were obtained for each factor using the survminer package in R studio. one-year mortality a). overall and by b). estimated Glomerular Filtration Rate (CKD-EPI, eGFR) <= / >20 ml/min/1.73 m2, and c). Combined Burden of Events score (CBOE) <=/>8.
Multivariate analysis was performed using backwards elimination. There was no co-linearity (>0.7) observed between the predictive factors.
AAV = ANCA associated vasculitis, GPA = granulomatosis with polyangiitis, PR3 = Proteinase-3 ANCA, eGFR = estimated Glomerular Filtration Rate (CKD-EPI), ml/min/1.73 m2, BVAS = Birmingham Vasculitis Activity Score, CYC = Cyclophosphamide, RTX = Rituximab, ENT = Ear, Nose and Throat, 95% CI = 95% Confidence Interval
There was no co-linearity (>0.7) observed between the predictive factors. N = 390 as one participant had missing age and six were missing induction treatment data. AAV = ANCA associated vasculitis, GPA = granulomatosis with polyangiitis, eGFR = estimated Glomerular Filtration Rate (CKD-EPI), ml/min/1.73 m2, 95% CI = 95% Confidence Interval
During the study period 55 patients (13.9%) died. Two- and five-year patient survival was 90.7% (87.7–93.9) and 76.8% (70.8–83.3), respectively (Figure 2a). Figure 2b and Extended data: Supplementary Table 57 summarise the factors associated with overall mortality. After controlling for age and other potential confounders, only eGFR at diagnosis and CBOE score were independently significantly associated with mortality. The CBOE mortality association was primarily driven by the cumulative infection score.
End-stage kidney disease (ESKD) developed in 73 patients (18.4%). 1-, 2- and 5-year actuarial renal survival was 85.1% (81.5–88.8), 83.3% (79.6–87.3) and 79.2% (74.4–84.4), respectively (Figure 3a). ESKD-free patient survival at 1-, 2-, and 5-years was 80.6% (76.6–84.7), 77.7% (73.5–82.1) and 63.9% (57.9–70.5), respectively (Figure 3b). At initial presentation 84 (21.1%) required renal replacement therapy, of whom 37 (44.4%) subsequently became dialysis independent. Of those who had renal involvement (86%), the median eGFR at diagnosis was 22 ml/min (IQR 11–42, Extended data: Supplementary Figure 5)7. Factors independently associated with ESKD risk included degree of renal impairment and having >50% globally sclerosed glomeruli on kidney biopsy (Table 4, model 2). usCD163 was measured at diagnosis in a subgroup of 103 patients, in whom usCD163 was tightly associated with ESKD, even after adjustment for eGFR (Table 4, model 3). The optimal diagnosis eGFR and usCD163 cut-points which maximally split the cohort into low and high-risk groups of ESKD were 12ml/min/1.73m2 and 740ng/mmol respectively (Figure 3c–d and Extended data: Supplementary Figure 6a–b)7. Patients with a ‘high’ (>740 ng/mmol) baseline usCD163 concentration were over five times more likely to develop ESKD, after adjusting for key confounders (Extended data: Supplementary Figure 7)7.
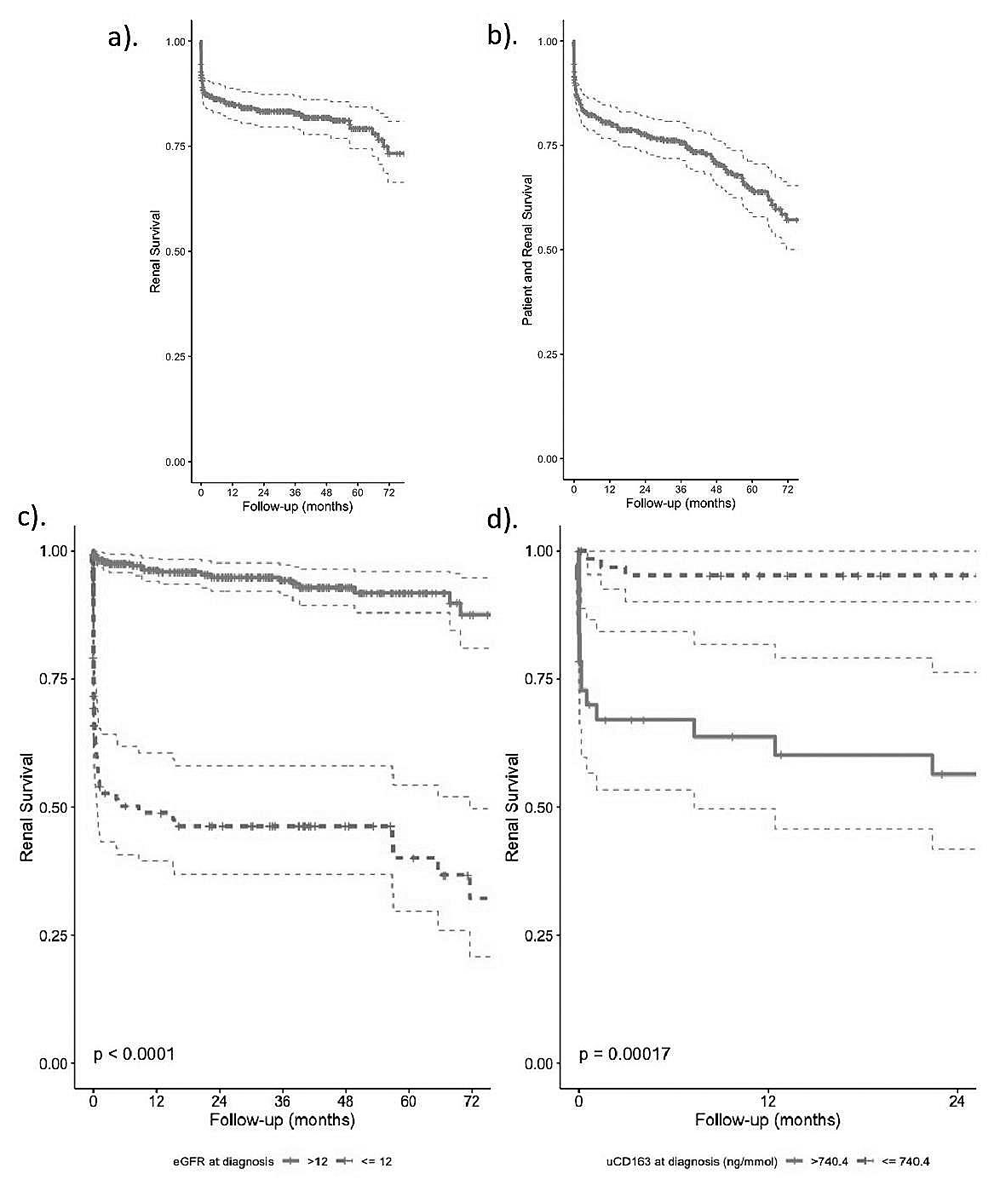
Optimal cut-points were obtained using the survminer package in R. a). Overall renal survival, b). ESKD-free patient survival, and renal survival stratified by c). estimated Glomerular Filtration Rate (CKD-EPI, eGFR) at diagnosis of <= / >12 ml/min/1.73 m2 and d). urine soluble CD163 at diagnosis (usCD163) of <= / >740.4 ng/mmol.
aModel 1 included baseline eGFR, adjusted for age at diagnosis, gender, AAV phenotype, ANCA serotype and the occurrence of a renal relapse (N = 397).
bModel 2 included baseline eGFR, adjusted for age at diagnosis, gender, AAV phenotype, ANCA serotype and Berden score on diagnostic renal biopsy (N = 244 as 153 participants did not have biopsy data).
cModel 3 included baseline uCD163, adjusted for baseline eGFR, age at diagnosis, gender, AAV phenotype and ANCA serotype (N = 103 as 294 participants did not have baseline uCD163 data).
There was no co-linearity (>0.7) observed between the predictive factors included in the multivariate models. *Missing data: uCD163 N = 294, Berden score N = 153. AAV = ANCA associated vasculitis, GPA = granulomatosis with polyangiitis, PR3 = Proteinase-3 ANCA, eGFR = estimated Glomerular Filtration Rate (CKD-EPI), ml/min/1.73 m2, BVAS = Birmingham Vasculitis Activity Score, RRT = Renal Replacement Therapy, 95% CI = 95% Confidence Interval.
| Hazard ratio (95% CI, p Value) | ||||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| Explanatory variable | Model 1a | Model 2b | Model 3c | |
| Age at diagnosis, years | 1.02 (1.00–1.04, p=0.054) | 0.996 (0.98–1.02, p=0.708) | 0.996 (0.97–1.02, p=0.709) | 1.01 (0.98–1.05, p=0.498) |
| Female (Ref: Male) | 0.68 (0.42–1.11, p=0.126) | 0.66 (0.40–1.10, p=0.110) | 0.65 (0.37–1.15, p=0.141) | 0.17 (0.05–0.62, p=0.007) |
| AAV Phenotype: Not GPA (Ref: GPA) | 2.78 (1.55–4.98, p=0.001) | 1.34 (0.62–2.91, p=0.456) | 0.94 (0.41–2.14, p=0.885) | 1.28 (0.30–5.38, p=0.739) |
| ANCA: Not PR3 (Ref: PR3-ANCA) | 1.87 (1.13–3.09, p=0.014) | 0.86 (0.45–1.64, p=0.654) | 0.69 (0.35–1.35, p=0.275) | 2.09 (0.58–7.57, p=0.263) |
| eGFR at diagnosis | 0.92 (0.89–0.94, p<0.001) | 0.92 (0.89–0.94, p<0.001) | 0.93 (0.90–0.96, p<0.001) | 0.97 (0.94–0.995, p=0.02) |
| Required RRT at initial presentation | 13.26 (8.01–21.96, p<0.001) | - | - | - |
| Renal relapse | 0.74 (0.30–1.84, p=0.518) | 0.57 (0.23–1.42, P=0.225) | - | - |
| Berden score on diagnostic renal biopsy (Ref: Focal) | ||||
| Crescentic | 3.10 (1.16–8.32, p=0.024) | - | 0.80 (0.27–2.36, p=0.683) | - |
| Mixed | 3.62 (1.42–9.19, p=0.007) | - | 2.13 (0.95–4.79, p=0.275) | - |
| Sclerotic | 12.14 (4.93–29.86, p<0.001) | 5.76 (2.61–12.71, p=0.001) | - | |
| uCD163 at diagnosis (µg/mmol) | 1.37 (1.13–1.68, p=0.002) | - | - | 1.76 (1.24–2.50, p=0.002) |
| Observations | 397* | 397 | 244 | 103 |
| Concordance | 0.87 | 0.84 | 0.80 | |
We describe the Rare Kidney Disease (RKD) national Irish registry at the 10th anniversary of its inception. This multicentre longitudinal cohort study aimed to characterise the Irish AAV cohort, describe their long-term outcomes and identify baseline predictors of these outcomes. The cohort comprises an ethnically homogeneous Caucasian population, with most displaying renal involvement and hence a slight MPA and MPO-ANCA predominance was observed. This contrasts to the GPA preponderance20–23 in most other European registries, which tend to have lower rates of renal involvement. Our cohort were typically older, in agreement with recent studies showing an older age at diagnosis for those with MPA21,24,25. We noted a slight male preponderance, consistent with a prior meta-analysis of EUVAS RCTs22, which differs from the equal gender split most commonly reported2,20,21,23). Notably, the diagnostic delay (median 9.4 weeks, IQR 4.3–25.6, N = 238) in our cohort was somewhat shorter than other reported series (median range 12–52 weeks21,26–29).
Patient survival in our real-world cohort was comparable to the international literature, predominantly based on RCTs, which ranges from 80–97%, 80–85% and 70–80% for one, two and five years respectively2,5,22,26,30,31). One may have expected lower rates given our relatively older cohort with frequent renal insufficiency. We too noted a steep decline in survival in the initial 90 days, when maximal concomitant disease activity and immunosuppression occurs. Infection was the primary cause of death, consistent with previous reports5,22,26,32,33). Multiple studies have demonstrated an inverse relationship between survival and the number of accrued infections, particularly in the first year31,34). In our study, the weighted infection and leucopenia scores, reflecting the accumulation of these adverse events, were the strongest predictors of one-year mortality. This highlights the growing need for personalisation of immunosuppression to simultaneously achieve disease control while avoiding unnecessary excess immunosuppression, in addition to steroid-sparing, borne out by the PEXIVAS trial35. It also serves as a key reminder to clinicians to be alert to early infections and to routinely assess the need for infection prophylaxis against Pneumocystis jirovecii (PCP), fungi and viruses, as well as timely influenza, pneumococcal, hepatitis B virus and SARS-CoV-2 vaccination, as advised in guidelines36. Notably, only one patient died from PCP in our cohort, which may signify the success of trimethoprim/sulfamethoxazole prophylaxis, which is cost-effective in AAV37.
Our study supports the previously observed association between increasing age, impaired kidney function and early mortality5,20,21,30,33,38–41). While most studies have previously attributed this to excess severe infection21,31,32,42,43, we observed that advanced age and renal dysfunction are independent predictors, after adjustment for adverse events. Renal impairment results in immune dysfunction44 and altered pharmacokinetics, limiting drug clearance and hence increasing the risk of infection and treatment toxicity45. Our findings further support the use of dosing nomograms for cyclophosphamide based on age and eGFR to reduce treatment-induced leucopenia, thereby reducing mortality32,45. Future prospective research should explore whether a similar approach is necessary for rituximab, rather than the current fixed or weight-based dosing regimens.
Our study validates the use of the combined burden of events (CBOE) score5, to predict early mortality in an independent real-world cohort. Building on the initial description, we identified 8 as an optimal cut-off score to stratify patients into low/high risk of early death. Like the original description, a score ≤8 was associated with <5% risk of death within the first year. This risk rose 16-fold with a score >8. Analogous to scoring BVAS and Vasculitis Damage Index (VDI) during routine follow-up, clinicians should consider monitoring the cumulative CBOE score. A rising score warrants active assessment and implementation of strategies to reduce further toxicity. We also confirmed that renal dysfunction severity is the strongest independent risk factor of the CBOE score, underpinning the critical importance of accounting for kidney function when prescribing immunosuppression5. We found no statistical difference in the risk of a high CBOE score between the cyclophosphamide and rituximab groups, in keeping with the findings of the RAVE trial46. Our findings emphasise that no induction regime is ‘safe’ and future research should focus on developing remission induction strategies that minimise toxicity, while maintaining similar efficacy.
Reported renal survival in AAV is variable, largely related to differing baseline patient and disease characteristics across cohorts. Our one-, two- and five-year renal survival estimates of 85.1%, 83.3% and 79.2% respectively are in line with other series26,31,47–50): 20–40% of AAV patients reach ESKD by 5–7 years. Aligned with our relatively short diagnostic delay, the baseline renal function (median eGFR 22 ml/min 1.73m2) in those with renal involvement was comparable to international benchmarks (median eGFR range 12–25 ml/min/1.73m2)51,52. Our results confirm the importance of baseline renal dysfunction26,31,32,47,49,53 and of histological evidence of renal scarring54 in determining ESKD risk. Contrary to prior series, we did not find an association between ANCA serotype50 or prior relapse47 and ESKD, in multivariate analysis. Our group has recently demonstrated the benefit of usCD163, a glomerular macrophage marker, as a non-invasive tool for the diagnosis of AAV renal relapse55. Another group highlighted its use in the prediction of doubling of serum creatinine in lupus nephritis56. We demonstrate, for the first time, the potential use of baseline usCD163 in predicting ESKD at diagnosis, even after adjustment for renal insufficiency. We identified a cut-off of 740ng/mmol to stratify patients into low/high risk groups. The risk of ESKD was five-times higher for participants with a baseline usCD163 of >740.4ng/mmol compared with those below this cut-point. Once validated in independent cohorts, these findings will contribute to the personalisation of immunosuppression to minimise ESKD risk – a costly outcome to patients and society.
The main strengths of our study include the well-characterised national AAV cohort, recruited using uniform classification criteria. Our cohort does not exclude patients with severe disease, EGPA nor older people – commonly excluded in RCTs – and thus serves as a source of real-world data, which is important as outcomes differ to those reported from RCTs57. The use of REDCap as the registry infrastructure, with standardised interoperable ‘instruments’ to enable uniform data collection is another key strength. Our registry formed the basis of the model EUVAS registry which is being applied in nascent vasculitis registries across Europe. This capability is in line with both the EUVAS6 and European Reference Network initiatives (http://rita.ern-net.eu/about-rita/mission-goals-and-objectives/) to align and integrate European registries, with the aim of improving both care and outcomes in rare disease.
We also acknowledge the limitations of our study. As with any observational research, missing data is always a challenge. For this reason, we were unable to include BVAS in multivariate models. Although all relevant specialties were represented, most participants were recruited through nephrology centres, reflected in the high degree of renal involvement, which limits the generalisability of our findings to non-renal populations. The strengths and limitations of CBOE score were previously discussed in detail5. Additionally, the CBOE score was not adjusted for prophylaxis use. Induction treatment was included as a categorical variable – the variation in intensity of same (denoted by cumulative dosing) was not available. Going forward, we aim to capture the intensity of induction immunosuppression in our registry.
In conclusion, we demonstrated that long-term outcomes from a real-world Irish cohort are similar to international standards. Our results highlight that despite improvement in outcomes over the last number of decades, treatment toxicity is an ongoing concern and individualisation of immunosuppression relative to disease severity and relapse risk remains a key unmet need. Our findings serve to remind clinicians that the accumulation of adverse events, both in the first year and beyond, particularly in older patients and those with renal insufficiency, is strongly associated with premature death and should be accompanied by increased monitoring and adjustment of immunosuppression to further limit treatment-related morbidity. The degree of renal impairment at presentation is also a key determinant in renal survival, hence early identification remains a critical goal. Baseline usCD163 shows promise as a biomarker for ESKD prediction. Future research should further explore this in the context of a multi-modal approach to personalisation of AAV treatment.
Written informed consent for publication of the patients’ details was obtained from the patients.
While the underlying patient data is pseudonymised, due to the rarity of ANCA-associated vasculitis, coupled with the identifiable nature of the data included in our analyses, it is not possible in practice to fully anonymise the dataset. Individuals could potentially be re-identified quite easily. Therefore, raw data must remain confidential and cannot be freely shared on an open platform. We would invite any potential research collaborations or data requests through the corresponding author, Professor Mark Little (mlittle@tcd.ie), on reasonable request, as agreed by participants in their written informed consent (detailed on page 3: https://www.tcd.ie/medicine/thkc/assets/pdf/RKD-Vasculitis-Patient-PIL-ICF-Version-5-07AUG19.pdf). Requests will be considered on a case-by-case basis. This approach is endorsed in a recent publication in The Lancet, from another large Irish longitudinal cohort study, TILDA58.
Zenodo: Extended data for ‘ANCA-associated vasculitis in Ireland: a multi-centre national cohort study’. https://doi.org/10.5281/zenodo.73429347
This project contains the following extended data:
Supplementary Table 1: Rare Kidney Disease Registry and Biobank recruitment sites.
Supplementary Table 2: Baseline characteristics of all vasculitis patients (recruited prior to 31st May 2021).
Supplementary Table 3: Breakdown of additional agents used for those patients receiving maintenance rituximab (n=80).
Supplementary Table 4: Causes of death within the first year of observation in the AAV cohort diagnosed since 2012.
Supplementary Table 5: Uni- and Multivariate Cox Proportional Hazards Models to investigate the factors associated with overall mortality.
Supplementary Figure 1: Inclusion and exclusion criteria for the AAV patients included in the analysis of long-term outcomes.
Supplementary Figure 2: Diagnosis confidence matrix.
Supplementary Figure 3: Frequency and distribution of Combined Burden of Events (CBOE) score within the first year from diagnosis.
Supplementary figure 4: Impact of the Combined Burden Of adverse Events (CBOE) on mortality.
Supplementary Figure 5: Distribution of eGFR at diagnosis (ml/min/1.73/m2) in those with renal involvement: overall and stratified by VINE status.
Supplementary Figure 6: Optimal cut-points of a). 12 ml/min/1.73m2 for eGFR at diagnosis and b). 740.4 ng/mmol for usCD163 at diagnosis were determined, using the survminer package in R to categorize patients into low and high risk of ESKD.
Supplementary Figure 7: Forrest plot depicting the findings of a multivariate Cox Proportional Hazards Model to investigate the factors associated with end-stage kidney disease. usCD163 is included as a binary factor according to the optimal cut-point, leading to slightly different hazard ratios compared to Table 4.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0)
The authors would like to thank all participants who are enrolled in the Irish Rare Kidney Disease and Biobank, without whom this research would not be possible. We would also like to acknowledge the support of the clinicians, nurses and coordinators in the Vasculitis Ireland network (VINE) (https://www.tcd.ie/medicine/thkc/vasculitis/), as well as all consultant and trainee nephrologists in Ireland who are integral to ongoing recruitment to the registry. We also acknowledge the contribution of the scientists in the Trinity Health Research Group Laboratory, including Emma Leacy, Aisling Ui Mhaonaigh and Amrita Dwivedi, who are fundamental to the processing of research samples.
Members of the RKD consortium:
Tallaght Hospital: Mark Little*, Peter Lavin**, Catherine Wall**, George Mellotte**, Ronan Mullan**, Jennifer Scott**, Ted Fitzgerald**, Hannah O’Keefe**, Rachel Dilworth**, Claire Kennedy**, Limy Wong**, Eithne Nic an Ríogh**, Pamela O’Neill#, Vicki Carr#
St James’ Hospital: Niall Conlon*, Brenda Griffin*, Donal Sexton*, Caroline Kosgei#
Mater University Hospital: Yvonne O’Meara*, Eoghan White**, Stephen Mahony**
St Vincent’s University Hospital: Eamonn Molloy*, John Holian**
Galway University Hospital: Matt Griffin*, David Lappin**, Conor Judge**, Sarah Cormican**, Blathnaid O’Connell**, Michelle Clince**
Limerick University Hospital: Liam Casserly*
Cork University Hospital: Michael Clarkson*, Michelle O’Shaughnessy**, Alyssa Verrelli**, Sinead Stoneman**, Fergus Daly**, Laura Slattery**, Aisling Murphy#
Beaumont Hospital: Mark Little*, Declan De Freitas**, Peter Conlon**, Mark Denton**, Carol Treanor**, Colm Magee**, Conall O Seaghdha**, Paul O’Hara**, Susan McGrath**, Brona Moloney**, Dean Moore**, Dearbhla Kelly**, Mary McCarthy**, Tamara Wanigasekera**, Ayanfeoluwa Obilana** Dervla Connaughton**
*Principal Investigator; **Co-investigator; #Study Coordinator
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Glomerulonephritis, ANCA associated vasculitis
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Renal autommune diseases, glomerulonephritis, ANCA-associated vasculitis, lupus nephritis, membranoproliferative glomerulonephritis
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 01 Dec 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with HRB Open Research
Already registered? Sign in
Submission to HRB Open Research is open to all HRB grantholders or people working on a HRB-funded/co-funded grant on or since 1 January 2017. Sign up for information about developments, publishing and publications from HRB Open Research.
We'll keep you updated on any major new updates to HRB Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)