Keywords
COVID-19, vaccines, post-COVID-19 condition, PCC, long COVID
This article is included in the Coronavirus (COVID-19) collection.
COVID-19, vaccines, post-COVID-19 condition, PCC, long COVID
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was first identified in Wuhan, China in late 2019. The virus is the aetiologic agent of coronavirus associated infectious disease (COVID-19) and has caused an estimated 5.9 million deaths worldwide1.
The symptoms of COVID-19 include dyspnoea, cough, fever, anosmia, dysgeusia, diarrhoea, headache, coryza and myalgia2. Risk factors for severe disease include unvaccinated status, age, cardiovascular disease and immunocompromised person3. COVID-19 is also associated with significant morbidity. Studies suggest an increased risk of subsequent cardiovascular and pulmonary disorders including myo-pericarditis, ischaemic heart disease, cerebrovascular disease, dysrhythmias and thrombo-embolic disease4. In addition, many patients with COVID-19 suffer persistent symptoms long after initial infection.
Post-COVID-19 condition (PCC), often referred to as post-acute sequelae of COVID-19, or ‘long COVID’, affects from 30 to 90% of individuals six months after index infection5. Symptoms of PCC vary but can include chest pain, fatigue, cognitive dysfunction, dyspnoea, cough and disturbance in taste and smell6. Cohort studies have even identified distinct phenotypes of symptoms of including pain and cardiovascular domains7. Studies have also identified significant neuro-cognitive dysfunction in recovered COVID-19 patients, such as episodic memory impairment8, incident psychiatric disorder9 and persistent anosmia/hypogeusia10.
The pathophysiology of PCC is not fully understood. Cardiopulmonary testing in patients with PCC has demonstrated impairment in gas exchange11. Other studies have identified immune dysregulation12 and vascular dysfunction13 as possible pathways. Given the high incidence of PCC, funding bodies have increased research spending in this area, including the NIH which will invest over $1 billion through the Recover study14.
Vaccination for COVID-19 has been demonstrated to reduce the risk of hospitalisation and death, including against more recent variants15. Following the roll-out of the vaccination, there were reports of improvements in patients with PCC following vaccination. One study from the UK suggested that in patients with PCC, subsequent vaccination led to a reduction in symptoms at follow-up16. In addition, studies have reported a reduced likelihood of prolonged symptoms in vaccinated individuals17,18.
In addition to inflammatory, micro-vascular and other explanations for PCC, viral persistence has been touted as a plausible cause of PCC. Prolonged viral shedding in stool and respiratory tract has been demonstrated in patients with PCC19,20. Vaccination may play a role in early viral clearance and thus decrease the risk of PCC.
PCC is an urgent public health crisis. Vaccination is an acceptable, low-risk intervention that has already demonstrated efficacy at reducing the mortality in COVID-19. There is a need to further understand the additional benefit of reducing the risk of PCC. Also, the potential role of vaccination as a treatment among people with PCC warrants investigation.
World Health Organisation (WHO) established a Guideline Development Committee (GDC) to develop a living guideline for clinical management of COVID-19. To support the development of the new guideline, we report a rapid review of the effectiveness of COVID-19 vaccines for the treatment of people with PCC.
Registration: The rapid review followed the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) checklist21,22 and is informed by the rapid review guidance provided by the Cochrane Rapid Reviews Methods Group23. The protocol was registered in PROSPERO (International prospective register of systematic reviews) (CRD42022330821) on 20th June 2022. Compared to a full systematic review approach, in this rapid review, we searched two databases and two trial registration platforms as this review was commissioned to inform WHO interim guideline development and had to be produced within a tight timeline (12 weeks). However, two independent review authors screened citations, and extracted data of included studies.
Criteria for considering studies for this review
Population: We considered patients diagnosed with PCC. As per WHO, “Post-COVID-19 condition occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19 with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis. Common symptoms include fatigue, shortness of breath, cognitive dysfunction but also others and generally have an impact on everyday functioning. Symptoms may be new-onset following initial recovery from an acute COVID-19 episode or persist from the initial illness. Symptoms may also fluctuate or relapse over time”6. No minimal number of symptoms are required for the diagnosis; though symptoms involving different organs systems and clusters have been described. A full list of described symptoms and related definitions included in the WHO-led Delphi consensus statement6 can be found in the Extended data22.
Studies that included patients with PCC with other patient cohorts were excluded unless participants were included as part of a randomised trial using stratified randomisation and reporting results of patients with PCC separately. Studies focused on evaluate the effectiveness of vaccination in the prevention of PCC were excluded.
We recognised that studies may investigate the effects of vaccines in patients identified/classified to have PCC or ‘long-COVID’, where such patients do not strictly meet the WHO definition of PCC provided above (e.g., the condition occurring two vs. three months from the onset of the initial infection). To ensure comprehensiveness in identifying any potentially relevant evidence on this novel disease and to inform interim guideline development, such studies were intended to be included in this review version, and participant characteristics, including definition of a PCC used in each study, were detailed clearly. We planned to categorise and synthesise studies separately based on how they define a PCC (i.e., different PCC patient populations). This decision might be revisited for any future review update that may be initiated based on the expanding evidence base.
Intervention:
We considered COVID-19 vaccines as a therapeutic agent in people with PCC, regardless of whether administered as part of the primary series or as boosters. We considered both approved vaccines and investigational vaccines.
Comparisons:
We considered any of the following comparisons:
1. COVID-19 vaccine compared with no vaccine, placebo, or usual care.
2. One vaccine (vaccine A) with another (vaccine B)
3. Same vaccine in different doses and/or different time points between infection and vaccine receipt
Outcomes: The core outcome set for PCC24 was used in our review and is given below:
Physiological/clinical outcomes
1. Cardiovascular functioning, symptoms and conditions
2. Fatigue or Exhaustion
3. Pain
4. Nervous system functioning, symptoms and conditions
5. Cognitive functioning, symptoms and conditions
6. Mental functioning, symptoms and conditions
7. Respiratory functioning, symptoms and conditions
8. Post-exertion symptom
Life impact outcomes
Survival
Recovery
Other
We intended to include the above outcomes at any assessment time point.
Type of studies: We considered studies as per Cochrane Effective Practice and Organisation of Care guidelines25. Randomised trials, non-randomised trials, controlled before-after studies, and interrupted time-series studies were considered. The decision to include a range of study designs in addition to randomised controlled trials is driven by the need to synthesise available knowledge while many trials are currently lacking or are in progress. This decision will be revisited in any future review update.
Type of publications: We considered English language papers, available in full text and published in a peer-reviewed scientific journal or on preprint servers. Trial registration with or without published results was also considered for inclusion. All ongoing studies identified, including study protocols and registered trials, will be considered for any future review update. We also searched the citation list of relevant systematic reviews of randomised trials, non-randomised trials, controlled before-after studies, and interrupted time-series studies.
We excluded the following types of studies/publications:
Animal and in vitro studies.
Modelling studies.
Systematic reviews and other evidence reviews (although these were used for backward citation tracking).
Opinion pieces, editorials, conference abstracts.
Conference abstracts were excluded based on a likely lack of sufficient information on the methods included to enable the risk of bias assessment.
Search methods for identification
Electronic database: The review team developed the search strategy, which includes an information specialist (KK). We adapted the search strategy from the NICE Guideline 188 (National Institute for Health and Care Excellence). We searched Medline (OVID), EMBASE (Elsevier), ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP). The search was conducted on May 11 and the search timeline was from January 2020 to May 2022. The complete search strategy has been provided in the Extended data22.
Data collection and analysis
Software: We screened citations using Rayyan26. Rayyan is an open-source web-based software platform for screening articles in systematic reviews.
Screening: The screening of citations was conducted in two phases. After deduplication, the title and abstracts of citations were screened for eligibility independently by two reviewers. Disagreements were resolved through a discussion with a third reviewer. The full text of all papers included, or where unclear as to whether the paper was relevant at title and abstract stage, were screened independently by two reviewers. Where necessary, disagreements were resolved through a discussion with a third reviewer. In addition, a third pharmacist reviewer was consulted where there was uncertainty about the eligibility of the intervention.
Data extraction: We extracted data independently by two reviewers onto a pilot-tested form. Data were extracted on publication year, author name, types of studies, sample size, study population characteristics (age, sex, ethnicity, geographic location, PCC characteristics/symptoms), vaccine product, vaccine platform type, vaccine dose, number of vaccine doses, interval between vaccine doses, and interval between preventative vaccination and COVID-19 infection, interval between diagnosis of PCC and vaccination treatment, follow-up duration and outcome including time point of outcome assessment.
Risk of bias assessment: We had planned that two reviewers would independently assess the risk of bias of all included studies using the nine standard criteria suggested by the Cochrane EPOC guidelines to assess the risk in bias all randomised trials, non-randomised trials, and controlled before-after studies27 i.e., random sequence generation, allocation concealment, similar baseline outcome measurements, similar baseline characteristics, and incomplete outcome data, knowledge of the allocated interventions adequately prevented during the study, protection against contamination, selective outcome reporting and other risks of bias.
For interrupted time-series studies, we had planned to assess the intervention independent of other changes, the shape of the intervention effect pre-specified (e.g., a priori hypothesis on how the intervention would impact on the outcome if it were effective (immediate/lagged/temporary effect on level and/or slope of outcome)), intervention unlikely to affect data collection, knowledge of the allocated interventions adequately prevented during the study, incomplete outcome data adequacy, selective outcome reporting and other risks of bias. However, the risk of bias was not assessed as there were no completed studies.
Measures of treatment effect: We planned to calculate the risk ratio (RR) and corresponding 95% confidence interval (95% CI) for dichotomous data. For continuous data, we planned to extract the mean and standard deviation (SD). When the same outcome(s) was/were measured on the same scale or can be transferred to the same scale, we planned to calculate the mean difference (MD) on the original scale. When studies used different scales to measure the same outcome, we planned to calculate the standardised mean difference (SMD) and corresponding 95% CI for continuous outcomes.
Data synthesis: We had planned to conduct a random-effect model meta-analysis had we identified enough articles with similar interventions, populations and outcomes. We had chosen a random-effect model meta-analysis assuming heterogeneity in the intervention, participants and outcomes of included studies. We had planned to use the I2 statistic to measure the degree of statistical heterogeneity among the trials in each analysis. Where meta-analysis was not possible, we had planned to provide a narrative synthesis informed by the Synthesis Without Meta-analysis (SWiM) guidelines28. We had also planned a descriptive tabulation of the results summarising the body of evidence per intervention-outcome pair if a meta-analysis is not feasible. If we had conducted a meta-analysis and there was enough data, we had planned to carry out subgroup analysis to explore heterogeneity. Subgroup analyses were planned to be conducted to explore the effect in different subgroups such as children, older people, and pregnant women. Potential subgroups we had identified were:
1. Age: (i) children (under 18 years) vs. adults (ii) adults vs. older adults (over 60 years) (iii) pregnant women vs not pregnant
2. Acute illness disease severity (i.e., not requiring hospital admission, requiring hospital admission, and requiring intensive care unit (as adapted from Townsend et al.29)
3. Vaccination: product, platform type, dose, and timing
4. Type of COVID-19 variant including predominant variant at the time of infection preceding development of PCC.
We had planned to assess publication bias if there were 10 or more studies in the meta-analysis using funnel plot and Egger’s test.
Sensitivity analysis: We planned to perform sensitivity analyses to assess the robustness of our findings and explore the impact of methodological issues on effect sizes. This would have involved restricting the analysis to (a) summary effects by different study designs i.e., randomised trials and other designs and (b) studies judged to be at low risk of bias.
Summary of findings: We intended to provide a summary of the findings table using the GRADE approach (Grading of Recommendations, Assessment, Development, and Evaluation)30. We planned to assess the certainty of evidence for each outcome based on the study limitations, inconsistency, indirectness, imprecision and publication bias.
We could not measure the treatment effect, perform a meta-analysis, sensitivity analysis, or prepare a summary of findings as there were no completed trials.
Results of the search: The PRISMA flow diagram31, shown in Figure 1, describes the study selection process. We identified 9611 citations from the database and the registry searches. After removing 2551 duplicate citations, we screened the title and abstract of the remaining 7060 citations. We excluded 6954 citations at this stage and reviewed the remaining 106 full-text citations. Of the 106 citations, we excluded 104 and included 2 ongoing trials. Of the excluded citations, 10 were not on PCC, 46 were on pharmacological interventions, 11 were on dietary supplements, 8 were on traditional Chinese medicine, herbal and ayurvedic medicine, 4 were on plasma and platelet therapy, 4 were on stem cell therapy, 1 was on phototherapy, 6 were in Russian, and 14 were excluded due to study design. The Extended data provides the complete list of excluded studies and trial registries22.
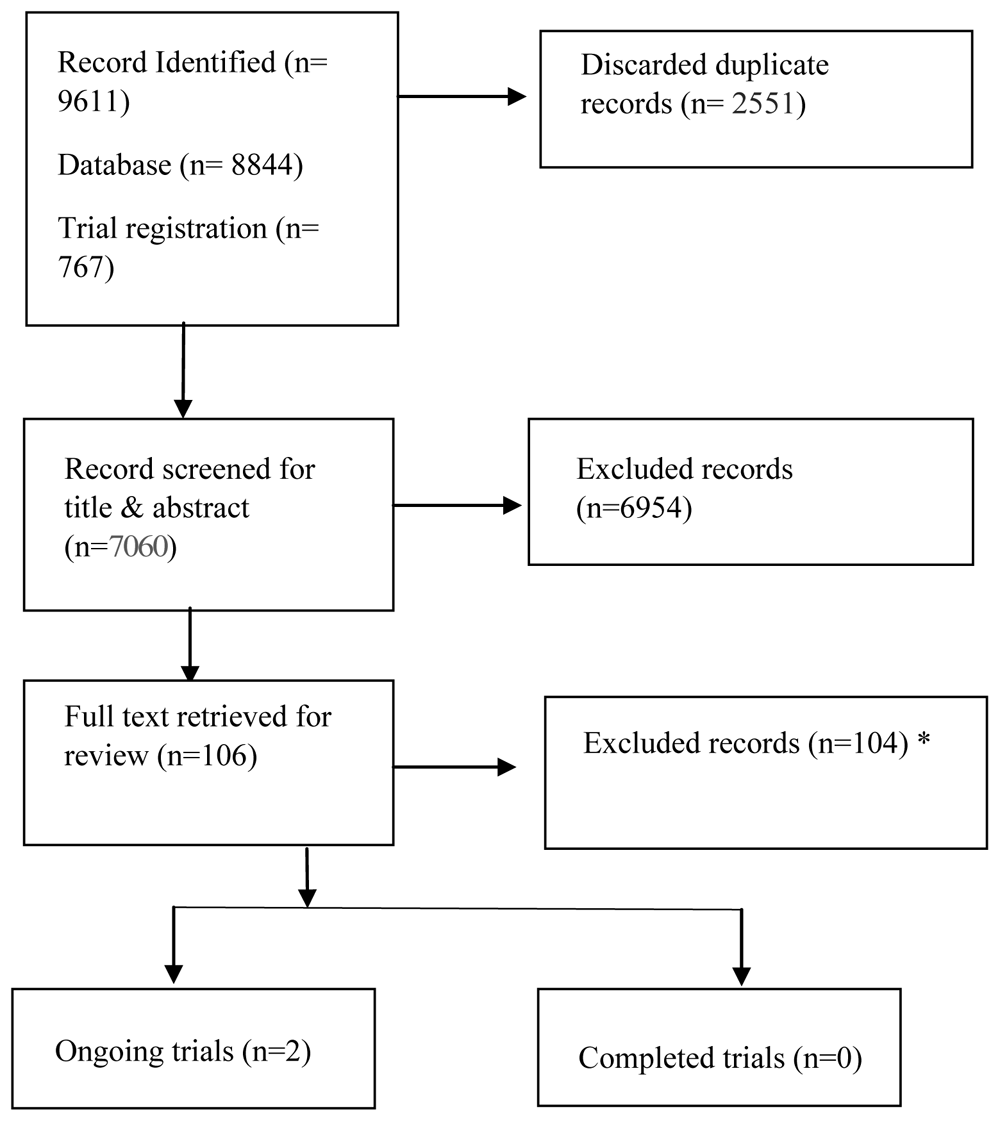
*Reasons for exclusion: Pharmacological intervention = 46, Not on post-COVID-19 Condition = 10, Dietary supplements =11, Traditional Chinese Medicine/ Herbal medicine/ Ayurvedic Medicine = 8, Plasma/ Platelet therapy = 4, Study design =14, Stem cell therapy = 4, Phototherapy =1, Article written in language other than English = 6.
Included ongoing studies: The two ongoing studies included in the review are described in the following sections.
Characteristics of the included studies: Both ongoing studies are randomised controlled trials. The trial of the COVID-19 mRNA vaccine (Comirnaty, marketed by Pfizer-BioNTech) is ‘double-blinded’ (authors do not state specifically who is blinded), and the CIMAvax-EGFA trial is open labelled. Both trials seek to include adult participants aged 18 years and older. The CIMAvax-EGFA trial plans to include 135 participants, and the trial of COVID-19 mRNA vaccine plans to include 776 participants who have not received any dose of any COVID-19 vaccine. Both trials were planned to start in 2021. The anticipated completion date of the CIMAvax-EGFA trial is January 2023, and the completion date of the COVID-19 mRNA vaccine trial is not stated. Table 1 describes the characteristics of the included ongoing trials.
Description of the interventions and comparisons: Both ongoing trials examine the effectiveness of therapeutic vaccines on PCC. In the CIMAvax-EGFA trial, participants in the intervention arm will receive 8 administrations of the vaccine. Each administration will have a dose of 2.4 mg of the active principle of the therapeutic vaccine CIMAvax-EGF® (rhEGF-rP64k conjugate) in 1.2 ml of the injection (vaccine in aqueous phase plus Montanide ISA 51 VG) intramuscularly. Participants will receive four doses during the induction phase with a dosing interval of 14 days. The rest of the four doses will be provided with a dosing interval of 28 days in the maintenance phase. In addition, the intervention group will receive supportive therapy. The control group will receive supportive therapy only, which may include steroids, bronchodilators, antibiotics in case of infection, hypotensive drugs in case of pulmonary hypertension, medicines for heart failure in case of cor pulmonale, oxygen therapy and/or pulmonary rehabilitation. Participants allocated to the intervention arm in the COVID-19 mRNA vaccine trial will receive the 2 doses of COVID-19 mRNA vaccine intramuscularly at an interval of 21 days, and the control group will receive a placebo.
Description of the outcome and measurements: The primary outcome of the CIMAvax-EGFA trial is the response to treatment measured through forced vital capacity (FVC) at days 63 and 182. The secondary outcome of this trial is the occurrence of any adverse events, adverse event description, duration and intensity of adverse events, causality relationship, gravity of adverse events, attitude towards drug, outcome of adverse events, measured after 6 months. The primary outcomes of the COVID-19 mRNA vaccine trial are safety and efficacy as measured through the change in the global score of the “COVID-19 Symptom Questionnaire” after 12 weeks of administration of the second dose. The secondary outcome is the change in the global score of the same questionnaire after 24 and 48 weeks after administration of the second dose.
Summary of main results: We conducted a comprehensive search and identified only two ongoing trials from trial registries. One of the ongoing trials (CIMAvax-EGFA) is due to be completed by January 2023 and the completion date of the COVID-19 mRNA vaccine trial is not stated.
Overall completeness and applicability of evidence: We conducted this review with a multidisciplinary team including evidence synthesis experts, an information and search specialist, clinicians, pharmacologists, and infectious disease specialists. To date, no trials or other controlled studies have reported an evaluation of the effectiveness of COVID-19 vaccines for treating people with post-COVID-19 condition.
Quality of the evidence: As we did not identify any published studies, we could not assess the quality of evidence according to the GRADE levels of evidence.
Potential biases in the review process: We searched the major databases and trial registration platforms. Screening and data extraction was done independently by two reviewers. A limitation of this review is that we considered articles published in English only. This means that it is possible that we missed relevant papers published in a language other than English. However, during our screening, we did not identify any non-English papers on effect of vaccines on post COVID-condition.
Implications for practice: There is currently an absence of high‐quality evidence evaluating the effectiveness of COVID-19 vaccines for treating people with post-COVID-19 condition. We identified two ongoing trials.
Implications for research: The absence of published studies and only two ongoing trials highlight the need for additional studies on the effectiveness of vaccines for PCC. We recommend that researchers consider including participants in trials who have PCC as per the WHO definition to minimise heterogeneity in the types of participants included across studies. The effectiveness of vaccination in people without PCC to prevent the development or reduce severity of PCC has been more commonly investigated. A recent retrospective study demonstrated that prior vaccination reduced the severity of the symptoms among hospitalised COVID-19 patients32. Another prospective cohort study using survey data demonstrated that participants receiving at least one dose of COVID-19 vaccine experienced fewer symptoms of post-COVID condition16. A recent rapid evidence synthesis reported 15 studies that explored the effectiveness of COVID-19 vaccines on “long COVID”33. They identified 7 studies that examined the effectiveness of the vaccination before infection in reducing the symptoms of long COVID, 7 studies that explored the effectiveness of vaccination after infection on long COVID and 1 study that examined both. However, all 15 studies are observational, and the authors identified the substantial heterogeneity in how “long COVID” was defined across the studies. These findings support the rational for robust trials on the effectiveness of COVID-19 vaccines on the treatment of post COVID condition. We recommend that trialists also use the available core outcome set for PCC in deciding which outcomes to measure and report in their trials. Given the presence of ongoing trials, we recommend that these trials be incorporated into a future update of this review.
All data underlying the results are available as part of the article and no additional source data are required.
Open Science Framework: Effect of COVID-19 vaccines for the treatment of people with post-COVID-19 condition: a rapid review. https://doi.org/10.17605/OSF.IO/UKDJ322.
This project contains the following extended data:
PRISMA checklist for ‘Effect of COVID-19 vaccines for the treatment of people with post-COVID-19 condition: a rapid review’. https://doi.org/10.17605/OSF.IO/UKDJ322.
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Partly
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
No
Are the conclusions drawn adequately supported by the results presented in the review?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Infectious Diseases
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 2 (revision) 11 Oct 23 |
|
|
Version 1 25 Oct 22 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with HRB Open Research
Already registered? Sign in
Submission to HRB Open Research is open to all HRB grantholders or people working on a HRB-funded/co-funded grant on or since 1 January 2017. Sign up for information about developments, publishing and publications from HRB Open Research.
We'll keep you updated on any major new updates to HRB Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)