Keywords
SARS-CoV-2, Saliva, SalivaDirect, Nasopharyngeal swabs, RT-qPCR
SARS-CoV-2, Saliva, SalivaDirect, Nasopharyngeal swabs, RT-qPCR
We thank the reviewers for their summary, comments and suggestions. We have now addressed the points raised in the different sections of the manuscript and we revised Figure 2 legend. Briefly, we added some information about the longitudinal study and the rationale behind exclusion criteria for saliva testing in the methods section (‘sample collection’ paragraph). We also added a comment regarding the thickness of the saliva samples and the care that should be taken to handle them in a laboratory setting to avoid cross-contamination (‘saliva sample processing’ paragraph). In the discussion section, we commented on the comparison of Ct values between NPS and saliva and on the diagnostic performance of PCR-based saliva compared to antigen testing (although antigen approaches were not tested in parallel in our cohorts). Finally, we expanded the reference list as appropriate to match the revised text and we corrected some typos.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus that rapidly spread across the globe in late December 2019 and was declared a pandemic by the World Health Organization (WHO) in March 2020 [press conference WHO 11th March 2020]. Containing the spread of SARS-CoV-2 has been a significant challenge worldwide mainly because both asymptomatic and symptomatic individuals can transmit the virus (Huff & Singh, 2020). A key approach to limiting cross-infection is robust testing and contact tracing. Currently, the gold standard for diagnosis is nucleic acid detection by reverse transcription quantitative PCR (RT-qPCR) (Adhikari et al., 2020). Nasopharyngeal swabs (NPS) were initially adopted as the preferred sampling procedure for SARS-CoV-2, due to established diagnostic practices for other respiratory infections (Li et al., 2013; Lieberman et al., 2009). However, NPS are invasive and may induce coughing and sneezing, increasing the risk of transmission to healthcare professionals (HCP) conducting the procedure (Kim et al., 2016). NPS discomfort can also be a barrier to repeated, frequent testing as swabbing can induce effects that can last up to 24 hours post procedure including epistaxis, headaches, earaches and rhinorrhea (Gupta et al., 2021).
Testing for SARS-CoV-2 in saliva mitigates many of the challenges associated with NPS sampling (Tan et al., 2021; Vaz et al., 2020; Wyllie et al., 2020). Although various different protocols for SARS-CoV-2 testing in saliva have been proposed, including colorimetric reverse transcription loop-mediated isothermal amplification (RT-LAMP) and lateral flow assays (Faustini et al., 2020; Lalli et al., 2021), RT-qPCR is the most common used modality (Caulley et al., 2021; Migueres et al., 2020; Teo et al., 2021) with a reported sensitivity between ~ 69 to 100% (Azzi et al., 2020; Kojima et al., 2020; Pasomsub et al., 2021; Skolimowska et al., 2020; To et al., 2020).
SalivaDirect is a nucleic acid extraction-free, cost effective and reliable method for detecting SARS-CoV2 which has been authorised for use by the FDA (Vogels et al., 2021, FDA press release 15th August 2020). Using SalivaDirect, specimens can be self-collected in a sterile sample tube without a viral transport medium. Proteinase K addition and a short heat-treatment step precede RT-qPCR analysis (Vogels et al., 2021). Initial studies using SalivaDirect reported a significant positive agreement (94%) between paired saliva and NPS samples obtained from a hospital cohort of 37 asymptomatic HCP and 30 COVID-19-positive inpatients (Vogels et al., 2021). In another study, matched saliva and NPS samples obtained from 30 individuals with COVID-19 illustrated a 88.2% concordance when using the SalivaDirect protocol (Rodríguez Flores et al., 2021).
Data comparing the concordance of ‘gold standard’ NPS and the SalivaDirect protocol for ongoing testing as part of an infection prevention and control programme are limited. In the present study, we set out to compare matching NPS and extraction-free saliva samples for detection of SARS-CoV-2 viral RNA via RT-qPCR, to assess the suitability of saliva as an alternative specimen for COVID-19 testing. We studied two key demographics – asymptomatic university students and hospital inpatients admitted with respiratory symptoms due to COVID-19 related illnesses.
This study was approved by the Ethics Committee of Royal College of Surgeons in Ireland (RCSI) (study code: REC202010011) and the National Research Ethics Committee for COVID-19 (20-NREC-COV-056). All subjects involved in this study provided written informed consent.
Research participants were recruited at two sites; a symptomatic patient cohort (n=127) was assembled at Beaumont Hospital, Dublin, Ireland and an asymptomatic ‘student’ cohort (n=181) at RCSI University of Medicine and Health Sciences, Dublin, Ireland. Recruitment took place during the period from November 2020 to March 2021 for the symptomatic population and in December 2020 and January 2021 for the asymptomatic student cohort.
Symptomatic individuals were inpatients at Beaumont Hospital who tested positive for SARS-CoV-2 on NPS-based diagnostic admission testing, carried out in the hospital’s clinical microbiology laboratory (using either the CerTest Biotec VIASURE SARS-CoV-2 real time PCR detection kit or Cepheid Xpert Xpress SARS-CoV-2). Patients were recruited to the research study post admission to hospital. Individuals in the symptomatic arm provided a research NPS sample, (collected by a trained professional), and a saliva sample (collected by passive drooling). The research NPS and saliva were collected on the same day. Asymptomatic students were recruited via an in-house screening programme at RCSI. NPS samples were collected by a trained professional with a saliva sample collected by passive drooling immediately afterwards. In both cohorts, exclusion criteria for participation to the study were any of the following activities conducted in the half hour prior to saliva sample collection: smoking, drinking any liquids, food, nasal sprays, tooth brushing and/or mouth washing. We set these as exclusion criteria as these activities might leave residues in the saliva (thereby influencing the samples quality of the sample and associated handling in the laboratory) or affect the samples viral load in the sample (Eduardo et al., 2021; Seneviratne et al., 2021)
NPS from the symptomatic cohort were placed in 2 ml of Viral Transport Media and sent to the CEPHR Laboratory using a biomedical courier. Samples were aliquoted into 2 ml cryovials and stored at -80°C until further use. Using 250 µl of the biobanked NPS, RNA was extracted using the automated platform for nucleic acid extraction EX3600 (Liferiver Biotech, Shanghai, China) according to manufacturer’s instructions, with a nucleic acid elution volume of 60 µl. Following RNA extraction, samples underwent RT-qPCR for SARS-CoV-2 with primers directed against the RNA-dependent RNA polymerase (RdRp) region of the viral genome, using the COVID-19 Genesig Real-Time PCR assay (Primerdesign Ltd, Hampshire, United Kingdom) on The LightCycler 480 PCR platform (Roche Diagnostics, Basel, Switzerland) with the following thermocycling conditions: 10 min 55°C, 2 min 95°C, 45 cycles of 10 sec 95°C/60 sec 60°C. Each run included a SARS-CoV-2 positive control (RNA), internal control, no-template control and a positive control of extraction (RNA from SARS-CoV-2 virus - 2019-nCoV/Italy-INMI1). Samples with quantification cycle (Cq) values below 40 cycles were defined as SARS-CoV-2-detected.
NPS from the asymptomatic cohort were processed in the COVID-19 Testing Lab at RCSI. RNA extraction for NPS samples was performed using a MagMax Viral/Pathogen II Nucleic Acid Isolation Kit (ThermoFisher Scientific), as per manufacturers’ instructions on a KingFisher Flex Purification system (model 5400630, ThermoFisher Scientific) instrument using 200 µL of NPS sample input. RT-qPCR followed the Centers for Disease Control and Prevention (CDC) protocol (Lu et al., 2020). Briefly, amplification of the SARS-CoV-2 nucleocapsid gene (N1 and N2) and internal control (RP) was performed using TaqPath 1-Step RT-qPCR Master Mix (4x, ThermoFisher Scientific) with the following sets of primers and probes: N1_F primer 5′- GACCCCAAAATCAGCGAAAT -3′ (500nM), N1_R primer 5′- TCTGGTTACTGCCAGTTGAATCTG -3′ (500nM), and N1_probe 5′-FAM-ACCCCGCATTACGTTTGGTGGACC -BHQ1-3′ (125nM); N2_F primer 5′- TTACAAACATTGGCCGCAAA -3′ (500nM), N2_R primer 5′- GCGCGACATTCCGAAGAA -3′ (500nM), and N2_probe 5′-Cy3- ACAATTTGCCCCCAGCGCTTCAG -BHQ1-3′ (125nM); RP_F primer 5′- AGATTTGGACCTGCGAGCG -3′ (500nM), RP_R primer 5′- GAGCGGCTGTCTCCACAAGT -3′ (500nM), and RP_probe 5′-Cy5- TTCTGACCTGAAGGCTCTGCGCG -BHQ1-3′ (125nM). The TaqPath RT-qPCR Master Mix (15 µL) was added to 5 µL of the RNA extracted from each NPS sample and run on a QuantStudio 7 (ThermoFisher Scientific) Pro Real-Time PCR with the following thermocycling conditions: 2 min 25°C, 15 min 50°C, 2 min 95°C and 45 cycles of 3 sec 95°C/30 sec 55°C. Cycle threshold (Ct) values lower than 40 was interpreted as detection of the gene and viral status of the clinical samples was called as per CDC recommendation (Lu et al., 2020).
All saliva specimens (i.e. from both the symptomatic and asymptomatic cohorts) were processed in the COVID-19 Testing Laboratory at RCSI using the SalivaDirect protocol (Vogels et al., 2021). Briefly, 50 µl of each saliva sample was added to 2.5 µl of proteinase K (50 mg/ml, ThermoFisher Scientific), vortexed and incubated at 95ºC for 5 minutes to ensure inactivation of the virus. The handling of the saliva samples (which were of a thick consistency) was performed with particular attention in order to avoid between-samples contamination. RT-qPCR amplification was performed using TaqPath 1-Step RT-qPCR Master Mix (4x, ThermoFisher Scientific), and the CDC N1 and RP sets of primers and probes as per SalivaDirect protocol at the following concentrations: N1_F primer (400nM), N1_R primer (400nM), and N1_probe (200nM); RP_F primer (150nM), RP_R primer (150nM), and RP_probe (200nM). The TaqPath RT-qPCR Master Mix (15 µL) was added to 5 µL of the “extraction-free” saliva sample and run on a QuantStudio 7 Pro Real-Time PCR with the following thermocycling conditions: 10 min 52°C, 2 min 95°C and 45 cycles of 10 sec 95°C/30 sec 55°C. Ct values lower than 40 were interpreted as detection of the gene and viral status was called as per SalivaDirect recommendation (Vogels et al., 2021).
In some cases, i.e. when the saliva and NPS testing results were not concordant, saliva samples were extracted using the MagMax Viral/Pathogen II Nucleic Acid Isolation Kit (ThermoFisher Scientific) following the protocol described above for NPS processing. This was a quality control step introduced to reduce the risk of false positive or negative results from saliva processed according to SalivaDirect protocol. RT-qPCR amplification of the SARS-CoV-2 ORF1 gene, N gene and S gene was performed using TaqPath 1‑Step Multiplex Master Mix (No ROX) (4X) and the TaqPath COVID-19 CE-IVD RT-PCR Kit as per manufacturers’ instructions. Briefly, 15 µL of the prepared mix were added to 10 µL of the RNA extracted from each saliva sample and run on a QuantStudio 7 Pro Real-Time PCR with the following thermocycling conditions: 2 min 25°C, 10 min 53°C, 2 min 95°C, and 40 cycles of 3 sec 95°C/30 sec 60°C. Ct values lower than 37 were interpreted as indicating the expression of that gene and viral status was called as per TaqPath COVID-19 CE-IVD RT-PCR Kit manufacturers’ instructions.
All statistical analyses were performed using GraphPad PRISM version 8.0.0 (GraphPad Software, San Diego, CA, USA). Sensitivity, specificity and 95% confidence intervals (CI) were calculated to assess diagnostic performance. Agreement between the NPS and saliva specimens for the virus detection ability was also assessed using Cohen’s Kappa (κ coefficient). Paired t-tests were used to compare the Ct values between NPS and saliva. Correlation between NPS and saliva Ct values were quantified using the Pearson correlation coefficient (Pearson r). All p-values were two-sided and p <0.05 was considered significant.
A total of 308 individuals, 181 asymptomatic students and 127 symptomatic patients, were included in this study (Table 1).
For the symptomatic patients, we report the maximum COVID-19 disease severity (as per WHO guidelines) reached during their hospital stay.
| Hospitalised patients (n=127) | Students (n=181) | ||
|---|---|---|---|
| Sex (%) | Male | 44.9 | 45.4 |
| Female | 37.8 | 52.4 | |
| Unknown | 17.3 | 2.2 | |
| Age (years) | Median (IQR$) | 68 (26) | 24 (3) |
| Clinical Phenotype (%) | Mild: 31.5 | Asymptomatic: 100 | |
| Moderate: 20.5 | |||
| Severe: 18.9 | |||
| Critical*: 9.4 | |||
| Unknown: 19.7 | |||
We first tested the correlation between NPS and saliva testing results in the symptomatic cohort. SARS-CoV-2 was detected in 86 of 127 patient NPS, while it was undetected in 41. Saliva testing in the same cohort indicated that 90 patients tested positive for SARS-CoV-2, while 37 tested negative. The sensitivity of saliva compared to NPS was 94.2% (95% CI 87.1-97.4%), while the specificity was 78.1% (95% CI 63.3-88%) in our symptomatic cohort.
We next tested the correlation between NPS and saliva in the asymptomatic cohort. Of 181 students, one tested positive both in NPS and saliva samples, while 180 tested negative in both. Sensitivity (95% CI 5-100%) and specificity (95% CI 97.9-100%) were therefore both 100% in this cohort.
The overall concordance between NPS and saliva testing is shown in Table 2. In the combined cohort, the sensitivity of saliva compared to NPS was 94.3% (95% CI 87.2-97.5%), while the specificity was 95.9% (95% CI 92.4-97.8%). Analysis of the concordance between the NPS and saliva specimens demonstrated an overall 95.5% accuracy for the saliva test relative to NPS and a very high level of agreement (κ coefficient = 0.889, 95% CI 0.833–0.946) between the two specimens. Nonetheless, we found 14 discordant samples between saliva and NPS. SARS-CoV-2 was detected in nine saliva samples but not detected in their matched NPS. On the other hand, viral RNA was not detected in five saliva samples but detected in the matched NPS. All 14 discordant samples belonged to the symptomatic hospitalised cohort. Ct values were >30 in 13/14 of these discordant samples and >35 in 6/14 samples.
| Nasopharyngeal swab | |||
|---|---|---|---|
| Positive | Negative | ||
| Salivadirect – Saliva | Positive | 82 | 9 |
| Negative | 5 | 212 | |
| Total | 87 | 221 | |
| Positive agreement = 94.3 % (95 % CI 87.2-97.5 %) | |||
| Negative agreement = 95.9 % (95 % CI 92.4-97.8 %) | |||
To further explore the discordant results, we extracted RNA from the 14 discordant saliva samples and retested the samples with the TaqPath COVID-19 CE-IVD RT-PCR kit. SARS-CoV-2 viral RNA detection was confirmed in 7/9 ‘false positive’ saliva samples, indicating these were actually true positives which the NPS test failed to detect. The remaining two saliva samples in which SARS-CoV-2 was undetected had Ct values ≥35 using the SalivaDirect protocol. All five ‘false negative’ also tested negative using the TaqPath protocol, indicating that in this case saliva testing failed to detect the virus which was instead detected by NPS testing. The RdRp Ct values from NPS from these samples were >30 using the Genesig Real-Time PCR assay.
We next compared the Ct values of the concordant positive saliva and NPS samples (Figure 1A) in the symptomatic cohort. N1 Ct values were reported for saliva samples according to SalivaDirect protocol, and RdRp Ct values for the NPS were reported, as it is the target gene of the Genesig Real-Time PCR assay. The overall mean and standard deviation (SD) Ct value for the positive NPS specimens and saliva samples was 26.36 (SD 7.03) and 26.49 (SD 6.04), respectively. The difference in mean Ct values (0.132) was not statistically significant (p=0.860). We also found a highly significant correlation between the Ct values of the positive saliva and NPS specimens (Pearson r = 0.475, p<0.0001, Figure 1B).
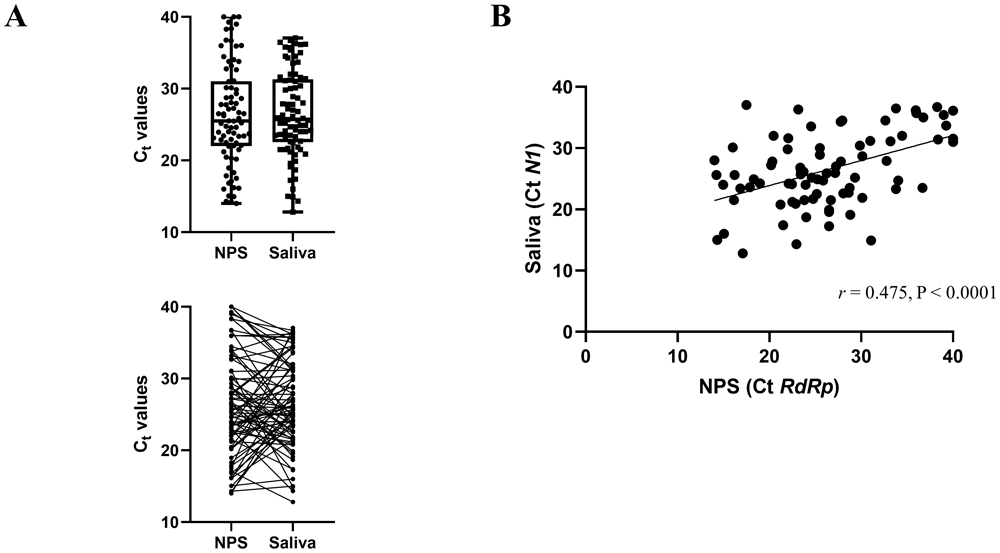
(A) The mean Ct values for saliva specimens are not significantly different than the mean for NPS specimens. The lines indicate samples from the same patient. (B) Correlation of Ct values between saliva (N1) and NPS (RdRp) PCR. The scatter plot shows the comparison of Ct values between the two methods.
Finally, we compared NPS and saliva test results obtained from the hospitalised symptomatic patients (127 individuals) with the results from their initial positive diagnostic SARS-CoV-2 swab, which was performed on admission to hospital. The average time between the initial diagnostic NPS and the paired (same day) research saliva and NPS was 4.73 days (95% CI 4.05-5.42) post initial diagnosis. As expected, the positive agreement of NPS and saliva testing decreased with time, but a similar pattern for saliva and NPS is notable (see Figure 2).
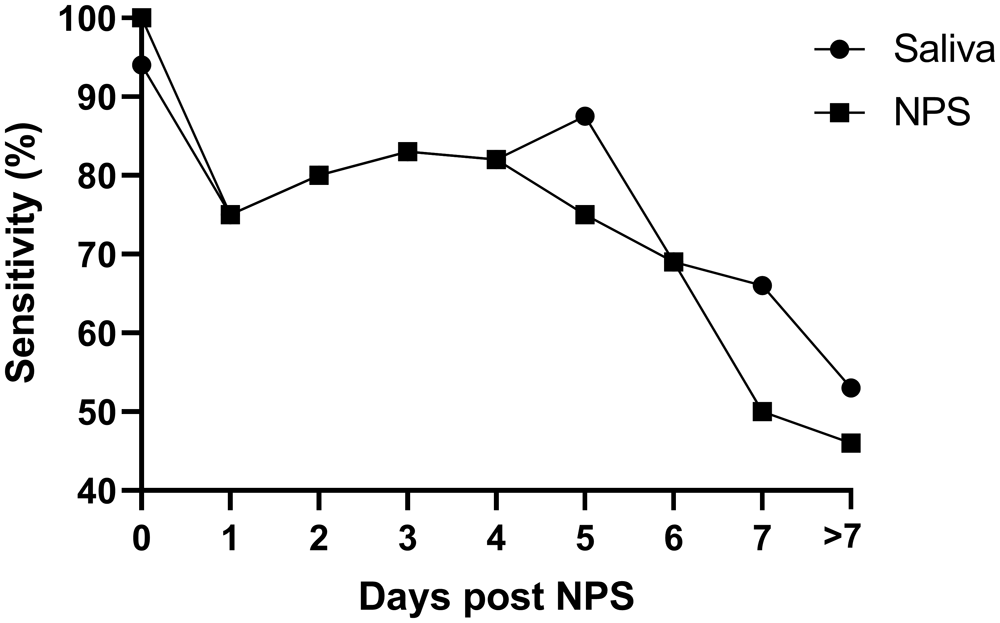
X axis: the delay (in days) between the positive NPS on hospital admission and the collection of the matching saliva and NPS sample. Matching NPS/saliva samples taken > 7 days post admission were pooled and reported as ‘>7’ (average of 11.53 days, 95% CI 10.02-13.03).
Use of saliva to detect SARS-CoV-2 represents a valid and accurate alternative to NPS sampling. Our results indicate a 94.3% sensitivity and 95.9% specificity of saliva when compared to a matching NPS taken on the same day in a combined cohort of symptomatic and asymptomatic individuals. Our results are consistent with the original publication of the SalivaDirect method, where the positive agreement was 94% in the hospitalised cohort, and sensitivity and specificity of saliva versus NPS in 3779 asymptomatic individuals were 89.5% and >99.9%, respectively (Vogels et al., 2021). A separate study reported a sensitivity of 88.2% of saliva samples assessed with SalivaDirect when compared to matching NPS samples taken from 30 COVID-19-positive individuals (Rodríguez Flores et al., 2021).
In samples where the virus was detected, we observed a strong correlation between viral gene Ct values across NPS and saliva samples, consistent with recent reports (Braz-Silva et al., 2020; Mahmoud et al., 2021). Saliva-positive samples often differ from NPS in terms of Ct values/viral load (Braz-Silva et al., 2021; Skolimowska et al., 2020; Wyllie et al., 2020), however in our hands NPS and saliva samples showed similar Ct values, possibly due to the fact that we tested for different viral genes for NPS (RdRp) and saliva (N1).
Although we found an overall accuracy of 95.5%, 14 samples from the symptomatic cohort were discordant between matching NPS and saliva samples, with five individuals testing positive by NPS only and nine by saliva only. The majority of these samples showed Ct values (of either RdRp for NPS or N1 for saliva) over 30, suggesting that a higher discordance between NPS and saliva testing results could be observed for high Ct values, as reported before (Kandel et al., 2020). When we extracted RNA from the 14 discordant saliva samples and tested them using an alternative PCR protocol, we found two ‘false positives’ in saliva. This confirmatory step in case of high Ct values from the SalivaDirect protocol could therefore be helpful to avoid false positives, which are proportionally greater in low prevalence settings (Basile et al., 2020; Healy et al., 2021).
In the hospitalised symptomatic cohort, we assessed the positivity rate in NPS, and saliva samples taken a variable amount of time after the first diagnosis. As expected, the positive agreement between the first diagnosis and the saliva or repeat NPS decreased over time, reflecting recovery and viral clearance. These findings are consistent with other longitudinal studies of COVID-19 testing (Smith et al., 2021; Wyllie et al., 2020). However, our results are the first to show that the drop in performance is consistent across saliva and NPS, thereby showing once again that the SalivaDirect accuracy is very similar to the gold-standard RT-qPCR from NPS.
Limitations of the current study include the presence of only one positive in the asymptomatic population which limits the value of the sensitivity calculation in that cohort. In addition, a more closely matched control group would have been beneficial in this study, as samples from a healthy student population were compared to the symptomatic hospitalised cohort. Lastly, the use of Ct values shows a trend of the viral load but does not allow exact quantification of viral copies/ml due to the absence of a standard curve included in the RT-qPCR analyses.
Overall, although the sensitivity and specificity are slightly lower, this work suggests that the SalivaDirect protocol represents a valid alternative to NPS. Other diagnostic assays are available including lateral flow antigen tests (LFAT). However, a pilot study applying LFAT in an asymptomatic population in the UK indicated a sensitivity of only ~49% when compared to gold standard NPS testing (Wise, 2020). More recent studies have reported sensitivities for rapid antigen testing compared to NPS-based molecular testing (i.e. RT-qPCR) of ~93% (Fernandez-Montero et al., 2021; Krüger et al., 2021; Van der Moeren et al., 2021) in the presence of low Ct values/high viral load, however these values dropped when including high Ct values/low viral load (71.4%, 79.8% and 82.2% in the three works, respectively). In our hands SalivaDirect sensitivity compared to NPS was retained for high Ct values by showing a sensitivity of 100% for Ct values lower than 30 and 94.3% overall. Most importantly, rapid antigen tests currently in use still require NPS as the sampling method, which is widely perceived as uncomfortable and could be a barrier for repeated testing. On the other hand, SalivaDirect employs saliva as input, which is less invasive and hence a more acceptable sampling method compared to NPS (Goldfarb et al., 2021; Kinloch et al., 2020) and whose collection does not require direct interaction between HCP and individuals. This offers several advantages, decreasing the potential for cross-infection as well as alleviating testing bottlenecks by decreasing the need for qualified HCP, personal protective equipment, and swab supply (Wyllie et al., 2020). Saliva can be self-collected, although individuals should be instructed in proper use of the self-collection tube, including its decontamination after saliva collection. These advantages are particularly relevant in the context of a surveillance programme where compliance rates play a crucial role in the long-term success of the screening initiative and individuals would benefit from a less invasive and accurate sampling method, easier to collect in a serial manner.
DRYAD: Concordance between PCR-based extraction-free saliva and nasopharyngeal swabs for SARS-CoV-2 testing, https://doi.org/10.5061/dryad.ksn02v74n (De Santi, 2021)
This project contains the following underlying data:
1. Ct_values_for_matched_NPS_and_saliva_samples_(asymptomatic_cohort).xlsx.This table shows the Ct values of N1 (both for NPS and saliva) in the samples belonging to the asymptomatic cohort (n=181 students).
2. Ct_values_for_matched_NPS_and_saliva_samples_(symptomatic_cohort).xlsx. This table shows the Ct values of RdRp (NPS) and N1 (saliva) in the samples belonging to the symptomatic cohort (n=127 hospitalised symptomatic individuals).
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
We are grateful to the students at the Royal College of Surgeons in Ireland and inpatients at Beaumont hospital who shared their samples and data for this study. We would like to thank our colleagues Jennifer Dowling, Richard Carroll, Stephanie Annett and Sudipto Das (School of Pharmacy and Biomolecular Sciences, RCSI) for their advice and support in setting up the COVID-19 Testing Laboratory at RCSI. We also thank Prof Samuel McConkey (Department of International Health and Tropical Medicine, RCSI) for his input in the discussion around this manuscript. Finally, we thank Zoe Yandle and Jonathan Dean (National Virus Reference Laboratory) and Prof Jonathan Ball (University of Nottingham) for their advices about setting up saliva testing at RCSI.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Epidemiology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Epidemiology, infectious diseases, respiratory infections
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 15 Oct 21 |
||
|
Version 1 30 Jul 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with HRB Open Research
Already registered? Sign in
Submission to HRB Open Research is open to all HRB grantholders or people working on a HRB-funded/co-funded grant on or since 1 January 2017. Sign up for information about developments, publishing and publications from HRB Open Research.
We'll keep you updated on any major new updates to HRB Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)