Keywords
Simulation, Practical Procedures, Phlebotomy, Patient Misidentification, Wrong Blood In Tube (WBIT),
Simulation, Practical Procedures, Phlebotomy, Patient Misidentification, Wrong Blood In Tube (WBIT),
The updated version highlights the steps in the metric which mitigate WBIT errors and expands on the importance of considering human factor science to examine barriers and facilitators to WBIT errors in healthcare organisations.
An error was noted in Table 4. Baseline characteristics 2017 pilot study and 2018 follow-on study groups and this was corrected.This did not affect the overall conclusion of the study.
Table 6. Mean number of blood tests collected by the interns, has been added.
The updated version provides further information on the methodology used for the qualitative component of the study.
See the authors' detailed response to the review by Paula H. B. Bolton-Maggs
See the authors' detailed response to the review by Richard M. Kaufman
Errors during sampling, labelling and transport to the laboratory (pre-analytical errors) are a common problem in the laboratory and account for up to 70% of all laboratory mistakes1. Frequent errors occurring in the pre-analytical phase include: i) identification errors, ii) errors in request procedures, iii) over- or under-filling of the specimen tube, iv) empty or missing tubes, v) contradictory demographic information on the tube and the request, (vi) ‘wrong blood in tube’ (WBIT)2. The most serious error, WBIT errors, occur when the blood is taken from the wrong patient but labelled with the intended patient’s details (mis-collected) or blood is taken from the intended patient and labelled with the wrong patient’s details (mislabeled samples)3. Guidelines exist on the correct practice of phlebotomy4–7. An observational study in 12 European countries demonstrated that compliance with phlebotomy procedures was low, with patient identification and tube labelling being the most critical steps requiring immediate action8.
Factors contributing to WBIT include incorrect patient registration9 and wrist band errors9, labelling remote from the patient10 failure to use positive patient identification3 and human errors. Human errors include slips, lapses , taking short cuts, distractions and omissions of essential steps . These ‘human factors’ are widely recognised and have been highlighted by SHOT and the National Hemovigilance office in Ireland11,12 Human factors are exacerbated by environmental issues such as fatigue, multitasking, short staffing and long shifts13.
The Testing the Utility of Collecting Blood Electronically (TUBE)14,15 found a lower incidence of WBIT in electronically labelled samples than manually labelled samples (1:3046 compared to 1:14,606, respectively). Furthermore, the sample rejection rate for samples deviating from labelling policy was 1:67 samples, with 1:26 of mislabelled samples being possible WBIT events, therefore justifying policies which do not use mislabelled samples for analysis or cross match. The benefit of electronically labelling samples has similarly been identified in other studies15
At our university hospital, the laboratory has been monitoring and recording details of the occurrence of WBIT and other sampling errors that result in the rejection of blood samples since 2010. Newly qualified doctors (interns) were frequently unaware of the hospital’s standard operating procedures relating to ordering of bloods from the laboratory through the electronic ordering system iSoft Clinical Manager System (iCM), and did not appreciate the critical importance of correct labelling of blood specimen bottles and laboratory forms. Much of this information was delivered either in a short induction lecture to the interns, learned in an apprenticeship style from their peers or self-taught. Efforts to reduce mislabelling errors have included educational sessions with the haemovigilance officer, educational leaflets at orientation, a zero tolerance policy for transfusion samples and educational campaigns to inform staff. These time consuming efforts, mitigate a peak in mislabelling experienced every July, but fail to reduce the ‘baseline rate’ of sampling errors. Since the introduction of an online request clinical management system (iCM) in the hospital identification errors have increased despite intensive standard training. This is a major concern. Video recordings of doctors performing phlebotomy identified practices with the potential to lead to incorrect labelling of blood specimens16 including printing labels before collecting blood and not labelling at the patient bedside. It is likely that since the introduction of the iCM system that doctors were not label at the bedside if under time pressure and labelled the tube only when they reach the label printer, therefore increasing the potential for a WBIT event.
Training and medical education can reduce the occurrence of pre-analytical errors including WBIT3,17–19 but is not sufficient to eradicate WBIT. This study proposes proficiency-based progression (PBP) simulation training as a solution to reduce pre-analytical errors including WBIT. PBP is an approach demonstrated to be more effective than traditional training models in procedural skills20–22. The study aims to compare the blood sampling error rate of interns who commenced work in Cork University Hospital (CUH) in July 2016 and did not receive PBP training in phlebotomy (historical controls), to PBP trained interventional groups who commenced work in July 2017 (intervention group 2017) and July 2018 (intervention group 2018) over three months.
This non-randomised controlled trial involved two phases, the first to develop the PBP training programme and the second to determine the effectiveness of the PBP programme to reduce blood sampling errors, primarily WBIT.
Phase 1: proficiency-based progression training programme development16. To design a new training programme, phlebotomy procedure metrics were characterised, guided by the methodological design outlined by Gallagher et al.23. We identified and defined 11 phases of the phlebotomy procedure. These 11 phases had 77-steps (metrics) for safe phlebotomy performance. The procedure characterisation focused on the correct procedure performance, patient safety and on identifying critical steps to avoid errors, including pre-analytical phase blood specimen errors and WBIT. These phases and metrics were then presented to a multidisciplinary Delphi panel of procedure experts, who unanimously concurred that they represented a comprehensive, quantifiable depiction of the procedure. Following the Delphi panel, the metrics demonstrated construct validity (mean inter-rater reliability 0.91). An expert panel established the proficiency benchmark at a minimum observation of 69 steps, with no critical errors and no more than 13 errors in total. A list of the 11 phases and the defined critical errors are illustrated in Table 116. Table 2 lists the key metrics which mitigate WBIT.
| Phase number | Procedure phase | Step Number | Critical Errors within phase |
|---|---|---|---|
| I | Introduction | ||
| II | If Crossmatch Required | 5 | Completes all shaded areas of the blood transfusion form |
| III | Goes to room where equipment is kept | 9 | Places closed (but not locked) sharps bin on tray |
| IV | Goes to patient | 19 | Requests permission to take blood |
| 22 | Checks name, patient identification number on identification wristband against the written instructions (or against group and hold/crossmatch form if applicable) | ||
| V | Ergonomics of procedure | 24 | Asks the patient if there is any particularly suitable vein and if one of their arms is unsuitable for venipuncture (ensures that none of the following are present: thrombophlebitis, lymphoedema, PICC line, renal fistula or a running IV infusion /TPN/ blood transfusion) |
| VI | Prepares equipment | 31 39 | Positions procedures tray with sharps bin within arms’ reach Puts on gloves (gloves are snug fitting with no overhang and are intact) |
| VII | Takes blood | ||
| VIII | Gets ready to remove needle | 50 51 | Release tourniquet while last blood tube is filling before removing needle from arm Once all blood tubes collected – disconnects last blood tube before removing needle |
| IX | Fixes patient up after procedure and labelling of blood tube | 60 61 | Writes down patient's name and date of birth or patient identification number onto the blood tubes using a pen before leaving bedside If mobile patient label printer is available at bedside, prints label and checks it* against the wristband. |
| X | Computer | 72 73 | Prints off patient’s labels for blood tubes after blood collection Checks name and date of birth/ patient identification number on labels against patient’s details written on the blood tubes if applicable. |
| XI | Tidies up and sends bloods off |
Phase 2: controlled trial. The second phase of the study aimed to determine if this bespoke PBP training programme could reduce the blood sampling and labelling error rate, including WBIT. The PBP training programme was delivered to the incoming interns in July 2017 and in 2018. The blood sampling error rate was monitored over three months, and compared to interns commencing work in July 2016 (historical controls). The study examined blood samples in the haematology department, including full blood counts and coagulation profiles. WBIT events are usually identified in these sample types when there is a large discrepancy with previous results.
Qualitative analysis took place during mentorship of the interns while performing clinical duties to investigate which factors were contributing to blood sampling errors and to inform the training programme for the next phase of the study in July 2018.
The study took place in a university teaching hospital with 800 beds. Participants were interns who commenced work in July of each year for a three-month rotation, recruited at induction training in the hospital.
Control group. The control group was comprised of 45 interns who were commencing work for the first-time following graduation in July 2016. The control group data had been collected prospectively as part of routine clinical practice. This group had received traditional phlebotomy training as medical students in their third medical year on two occasions and once in the final year of medical school, comprising a phlebotomy guide, training videos, and a practical training session in the clinical skills laboratory.
2017 pilot study group and 2018 follow-on study group. The intervention group consisted of 45 interns who were commencing work for the first-time following graduation in July 2017 and 46 interns commencing work in July 2018. All the interns commencing work in the hospital in these years consented to participate. They were invited to the training as part of their induction but participation in the trial was optional. The intervention, in the form of a PBP training programme, was given to the interns on the commencement of their employment in the hospital. The group gave written informed consent for enrolment into the controlled trial.
The interns who were commencing work in July 2017 first completed an online training module, comprising of a video of the correct process of performing bloods in the hospital. In the second component, the interns attended face-to-face training. This consisted of a short motivational introductory talk from a consultant haematologist and a laboratory scientist to outline the importance of following the correct procedure and the consequences of errors. The interns worked in groups of three, with one person acting as a patient, a second person marking according to the metric, and a third person taking bloods. A tutor was assigned to each team. Tutors included experts in phlebotomy in the hospital and comprised of phlebotomists, nurses with expertise in IV cannula education, senior lecturers from UCC experienced in providing teaching in phlebotomy skills and doctors who had participated in the metric development. A one hour session was provided before the teaching to ensure the teaching was standardised. Each person had to perform phlebotomy on model IV arms on a simulated ward to the proficiency standard of performing at least 69 steps with no more than 13 errors occurring and no critical errors allowed, to graduate from the course. The third phase of training occurred once the interns had commenced work. The interns were observed performing phlebotomy on patients on the wards to ensure they continued to achieve the proficiency benchmark in real time. The eLearning module and the simulation training on the training ward was completed before work commenced in the hospital. However, mentorship on the wards was completed in the first month following commencement of work in the hospital while data collection was ongoing. The eLearning module was made available on www.hseland.ie.
All 124 interns were trained in July 2018 and due to work in the hospital during the 2018/2019 rotation, which led to the 2018 group having fewer tutors available; the intern-to-tutor ratio was therefore much higher than in the 2017 intervention group. The ratio changed from three students per tutor in 2017 to 6–12 students per tutor for some sessions in 2018. Additionally, in 2018, each intern was provided with feedback on any error that occurred in the transfusion or haematology laboratory at the end of each month.
Cognos was used to interrogate the laboratory information system, APEX, for pre-analytical phase blood specimen errors and WBIT. Blood samples were collected on the general wards and the emergency department but did not include outpatient samples. Of note in 2016 interns took only 6% of the bloods on the wards in the three month period analysed. The electronic ordering software ICM system records the person who prints the label placed on the tube after taking the test. A search on the ICM system provided a list of each blood test collected in the three-month period, including the healthcare practitioner who collected the test and the patient identifier number, to link to the rejected samples in APEX. By matching the searches, this provided a list of the persons who obtained the rejected samples and a list of how many blood tests were taken by the interns over three months. Descriptive statistics were performed on the type and rate of errors. WBIT was the primary outcome. Secondary outcomes were all sampling errors (including WBIT). These included over- or under-filling of the tube, clotted samples, haemolysed samples, incorrect tube type received, no specimen received, and miscellaneous errors. Logistic regression analysis was used to examine the association between the odds for rejects in the 2016 control group to the intervention groups in 2017 and 2018 using Statistical Programme for Social Sciences (SPSS V24, IBM Corporation, 2016). To adjust for potential confounding factors, the month of the test and whether the test was taken on call or during normal working hours were included in the analysis. In a post-hoc analysis, we examined the potential clustering effect by intern in the logistic regression models. A p-value <0.05 was considered significant.
A process evaluation took place during mentorship on the wards. Field notes were recorded by the investigator immediately after observing the intern collecting blood on the wards, observing ease of access to equipment, the patient, the computer, the label printer, and any interruptions which occurred. Steps of the phlebotomy metric which required assistance or that were omitted were recorded. A standard form was developed to record the field notes. A semi-structured interview was performed at the end of training comprising of two open ended questions (See Table 3). The responses were recorded by taking notes and transcribed in excel. The notes were reviewed using a theoretical domain analysis framework24 by two reviewers. The results of this qualitative analysis were used to inform changes to the training programme in 2018.
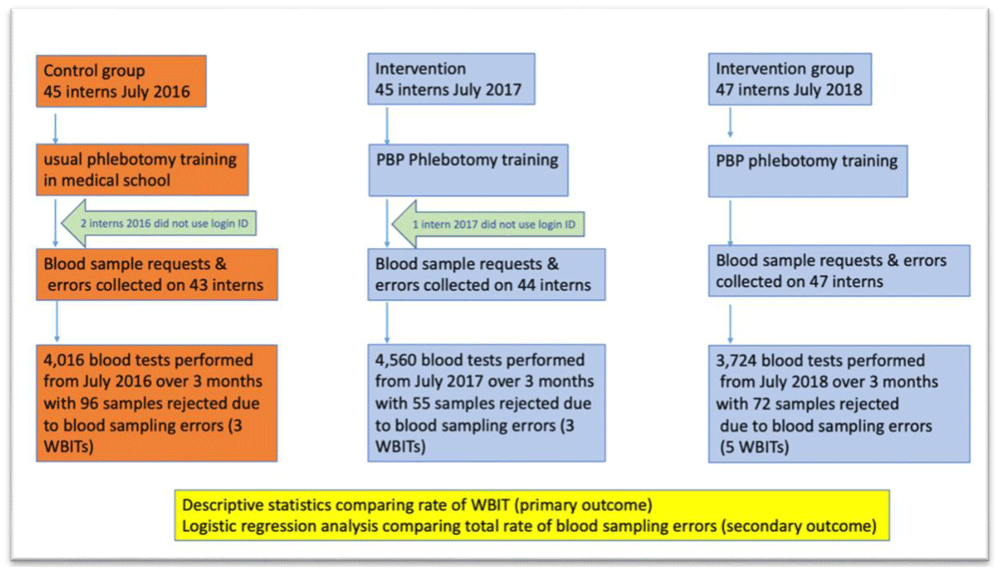
The baseline characteristics of the 2017 pilot study and 2018 follow-on study groups are provided in Table 4. Descriptive statistics are not available for the 2016 control group as they were not working in the hospital at the time the study started. Figure 1 provides a flow chart to illustrate the recruitment of interns and the analysis of blood tests which they collected during the trial. The mean number of blood tests performed by the interns in each year is described in Table 5.
*Data was not collected on historical controls but 20 of the 43 who collected bloods were male.
| Year | Average Number of blood test collected (min-max) | Standard Deviation |
|---|---|---|
| 2016 | 91 (10-228) | 50 |
| 2017 | 101 (2-262) | 52 |
| 2018 | 95 (26-224) | 44 |
There appeared to be an increase in the primary outcome, WBIT, (although the numbers detected are very small) from 0.7 per 1,000 in 2016 (three WBITs) and 0.66 per 1,000 in 2017 (three WBITs), increasing to 1.3 per 1,000 in 2018 (five WBITs). The absolute numbers make an interpretation of trends difficult. Each of the instances of WBIT was identified by the laboratory, but one of the WBITs in 2018 was identified when the doctor rang the laboratory to self-report that they had mislabeled the tube. It is possible that this type of event would have been undetected in 2016, unless there was a discrepancy with previous results available in the laboratory.
There were 4,016 blood tests collected by interns in the control group who did not receive PBP phlebotomy training from July 11th, 2016, to September 10th, 2016; 96 (2.4%) of the blood samples were rejected. For the same period in 2017, 4,560 tests were taken by PBP trained interns and 55 (1.2%) of the blood samples were rejected. In 2018, 3,724 tests were taken by PBP-trained interns and 72 (1.9%) were rejected. Table 6 describes the breakdown of errors that occurred.
Logistic regression analysis (Table 7) suggested that there was a reduction in the odds of test rejection in the 2017 pilot study when interns underwent PBP phlebotomy training, in comparison to the 2016 control group, and this difference was statistically significant (adjusted OR=0.50, 95% CI 0.36-0.70, p<0.001). The results for 2018 showed a 11% reduction in the odds of a blood sample being rejected in the PBP trained group in comparison to 2016 control group, but this was not statistically significant (adjusted OR=0.89, 95% CI 0.65-1.21, p=0.46).
During mentorship on the wards, interns were observed performing blood sampling in their usual clinical environment. Dialogue between the mentor and the interns was structured as a series of open questions during PBP training/mentorship. Of the 45 interns who attend for PBP training on the wards, observations were recorded for 40 interns. Analysis using a theoretical domain framework revealed four themes:
Environmental context and resources: Written instructions given to the interns on the ward were noted to be poor with only one patient identifier provided. Time delays were commonly caused by label printers not working (seven cases) and unavailability of computers (eight cases). Essential equipment such as Azowipes®, tourniquets or blood tubes, were frequently missing (21 cases). Interns reported occasional instances of phlebotomy in patients who were not wearing an ID band.
Emotion: Nursing staff support on the wards contributed to calm and safe phlebotomy performance, while there were frequent interruptions by bleeps and time constraints contributed to stress, and multiple technical errors. Several interns expressed nervousness while somebody was watching them perform phlebotomy.
Knowledge: Interns had an average of 5.8 errors. They required prompting to ensure the steps of the metric were followed correctly.
Social influences: The interns appeared to be influenced by their senior colleagues, some of whom felt that labelling at the bedside and printing labels at the computer located away from the patient after taking bloods was time consuming and unnecessary extra work.
This study demonstrated that interns who received PBP training in 2017 had significantly fewer samples rejected than in the control group. There was a 25% reduction in errors in 2017. A reduction in errors was recorded in 2018 (compared to historical controls), amounting to a 11% reduction in odds for a rejected sample which was not statistically significant. The diminished effectiveness is possibly multifactorial, including the lack of safety culture around mislabeling on the wards (senior peers did not put value on positive patient identification, did not label at the bedside and sometimes printed labels before attending the patient) and environmental stressors (including distractions such as bleeps, interruptions with requests to perform other tasks, time pressure and difficulty locating essential equipment). Due to difficulty recruiting and the large number of interns trained in July 2018, the ratio of tutors to students increased, with one tutor attempting to teach 6–12 students for some sessions. The enthusiasm around PBP training in the hospital was not as heightened in 2018, possibly due to familiarity and perceived lack of reduction in WBITs.
This study follows the methodology of previous research indicating the benefits of PBP training21,22. It is a novel technique using technology-enhanced learning and simulation. Previous educational strategies tend to follow didactic-style teaching to improve phlebotomy technique and often rely on self-reported questionnaires to determine effect25. Educational strategies have been shown to reduce but not eliminate WBIT17,18. Bar code systems that scan the patient’s wristband demonstrate a reduction in labelling errors and improve positive patient identification practices26,27. Many organisations, however, do not have sufficient resources to deploy these devices; the device is aimed primarily at transfusion sampling and requires proper training to be effective. Multiple interventions and feedback are likely to be more effective than single interventions, but the sustainability of improvements is not certain from previous research18. WBIT rates in mislabelled samples are estimated at 1.4%14, which is much higher than in correct samples, indicating that rejection of the sample due to any mislabelling event is indicative of an error-prone phlebotomy process that could have led to WBIT errors, and justifies the investigation of all blood sample errors to represent instances where there was a high risk of WBIT errors. This study demonstrates that, despite the introduction of comprehensive PBP training in phlebotomy, if the environment and process is error-prone, it is not possible to eliminate the risk of WBIT and other blood sampling errors. Healthcare organisations must adapt their systems to reduce distractions and tendency towards deviating from the correct process of phlebotomy which can lead to harm e.g. not performing positive patient identification, labelling the tube away from the bedside.
PBP training has robust evidence demonstrating a 40%-60% improvement in procedural performance20–22,28. This study examines the effectiveness of the intervention for a three-month period over two years. The study gives a clear insight into the sustainability of the project. The development and design of the project was multidisciplinary and involved all stakeholders in developing a training programme that was highly relevant.
While in many cases the use of historical data is not ideal, the thorough, systematic nature of this data, which has been documented systematically since 2010, allows us to be confident that the 2016 historical control group is representative.
The study has several limitations. The study did not have a sufficient sample size in order to examine the effect of the intervention on WBITs, the primary outcome of interest. One doctor in the 2017 group did not attend the final assessment and three of the doctors in the study were discovered to not be using their own ICM login, and therefore these participants could not continue the study.
Interns may have been negatively influenced by mentors who had not undergone PBP training and this could have undermined and weakened the potential impact of PBP training. There was a concern that, although the interns were trained to proficiency, they did not always follow the process when unsupervised as it took longer to perform.
The qualitative element of the study identified factors which could potentially increase the risk of WBIT, including patients not wearing identification wristbands, difficulty accessing essential equipment, insufficient hardware and stress. This provides an insight into the environment and context within the interns are expected to perform. These human factors were persistent in all years; however, it was not possible to measure the level of these over the three years. The qualitative component of the study highlights learnings of why it may not be possible to eradicate WBIT events if the interns are expected to work within an environment that does not promotes safety. Previous research describes the need to develop “resilient” healthcare organisations that are capable of identifying and adapting to potential vulnerabilities or threats to safety without the need for an incident or accident to occur and promotes the concept of a Safety-II approach for healthcare organisations to consider as an alternative to improve the quality and safety of their systems by ensuring more things can go right29. This study highlights the value in such an approach to support educational initiatives.
Given that the project was heavily promoted in the laboratory, it is possible that there was an increased awareness of WBIT, and this could have led to an increased detection rate amongst laboratory as well as ward staff. This detection bias has been described in previous studies involving WBIT, where errors increased despite the introduction of quality improvement initiatives30.
In conclusion, PBP training in phlebotomy can reduce blood sampling errors, but must take place in an environment that clearly acknowledges the importance of the training and the quality of the training must be properly resourced. Healthcare organisations must ensure that the process of performing bloods is organised in a way that promotes safety and make it easy to perform procedures correctly e.g. ensuring bedside label printers to promote bedside labelling.
This study was unable to demonstrate if PBP training in phlebotomy influences the incidence of WBIT due to an inadequate sample size and possible detection bias.
PBP training in phlebotomy can reduce blood sampling errors, but barriers to following the correct procedure on the wards must be considered and removed if possible.
Educational interventions alone are insufficient to reduce blood sampling, including WBIT, if the environment does not allow for a safe and efficient phlebotomy process, including the availability of appropriate hardware (especially bedside label printers).
This bespoke PBP training programme in phlebotomy can be adapted for use in other healthcare facilities to train healthcare practitioners at commencement of employment, following external validation.
The underlying data (anonymised) will be made available on request for bona fide researchers, including researchers outside of UCC. Approval for data sharing was not sought at ethics approval stage nor was it included in the information sheets and consent forms provided to participants. Also, the sample size is small, and this raises the risk of potential reidentification of participants. To request access to the data please email the corresponding author, Dr Noirin O’ Herlihy- 100312258@umail.ucc.ie
Figshare: The standard form used to collect field notes on the wards is available from https://doi.org/10.6084/m9.figshare.17242574.v1
This project contains the following data
- Standard form PBP on wards.docx
Data are available under the terms of the Creative Commons Atribution 4.0 International (CC BY 4.0).
We thank all the participating volunteers for their efforts and contributions including the laboratory at CUH and the healthcare practitioners who participated in the organisation and delivery of the PBP training programme in phlebotomy. We acknowledge Dr Patrick Kiely’s role in the development of the online eLearning programme.
NOH collected data, performed formal analysis, investigation, data curation, writing original draft, visualization, project administration, SG revised the manuscript, PH writing review and editing, investigation, RG writing review and editing, investigation, AK formal analysis and writing review and editing, MR data curation, writing review and editing, AG conceptualization, funding and acquisition, supervision. methodology, formal analysis writing review and editing, MC conceptualization, funding and acquisition, supervision. methodology, formal analysis writing review and editing,
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
References
1. Passwater M, Huggins YM, Delvo Favre ED, Mukhtar F, et al.: Adding Automation and Independent Dual Verification to Reduce Wrong Blood in Tube (WBIT) Events.Am J Clin Pathol. 2022; 158 (2): 212-215 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Transfusion Medicine, components storage and processing, massive transfusions for trauma ( adult and pediatrics, PPH), quality improvement.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Haematology, blood transfusion, patent safety, human factors, haemovigilance
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Transfusion medicine
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Robinson S, Harris A, Atkinson S, Atterbury C, et al.: The administration of blood components: a British Society for Haematology Guideline.Transfus Med. 28 (1): 3-21 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Haematology, blood transfusion, patent safety, human factors, haemovigilance
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 20 Dec 21 |
read | read | |
|
Version 1 22 Jul 21 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with HRB Open Research
Already registered? Sign in
Submission to HRB Open Research is open to all HRB grantholders or people working on a HRB-funded/co-funded grant on or since 1 January 2017. Sign up for information about developments, publishing and publications from HRB Open Research.
We'll keep you updated on any major new updates to HRB Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)