Keywords
lutein, zeaxanthin, diet, cognition, cognitive development, vision, children
This article is included in the Maternal and Child Health collection.
lutein, zeaxanthin, diet, cognition, cognitive development, vision, children
The dietary carotenoids lutein and zeaxanthin preferentially accumulate in neural tissue including the central region of the retina (macula) where they form macular pigment (MP). MP’s constituent carotenoids have been studied extensively for their role in eye health and in the prevention of retinal disease such as age-related macular degeneration (AMD) and, more recently, glaucoma1,2. Within the eye, the protective role of MP appears to relate to its light-filtering, antioxidant, anti-inflammatory and neuro-protective properties3,4. These carotenoids are also found in several specific regions in the brain including the frontal, occipital and temporal cortices, hippocampus and cerebellum from prenatal development into old age5–8. Retinal MP can be measured non-invasively and used as a surrogate measure of brain lutein and zeaxanthin status5. The selective accumulation of these carotenoids in the brain suggests that they may have a specific role within the central nervous system.
So far the beneficial effects of lutein and zeaxanthin have been primarily studied in relation to older age, due to the antioxidant, anti-inflammatory and neuroprotective properties of these carotenoids and the fact that oxidative stress and inflammation have been implicated as pivotal components of age-related visual and cognitive decline9–11. Indeed, higher levels of MP in the diet, serum, eye and in the brain later in life have been shown to be associated with better cognition12–17. At the same time, these compounds are present in the eye and brain from an early age, suggesting that they have an important biological role throughout life8,18,19. The possible importance of these carotenoids for cognitive development is indicated by the selective accumulation of lutein and zeaxanthin within the developing infant brain8. Lutein, in particular, is the predominant carotenoid in the brain tissue from infancy8. Despite constituting just 12% of overall infant carotenoid intake, lutein has been shown to account for 59% of total brain carotenoids8. Additionally, the relative contribution of lutein to total carotenoids is almost two-fold greater in the infant brain than in adults, accounting for 59% vs. 34%, respectively8,20. Lutein and zeaxanthin’s concentrations in the eye are still 2-3 times higher than in the brain5. Nevertheless, such selective concentration of lutein, and the multitude of effects it has on neural cell viability, suggests that it may play a key role in the early processes of neural and hence cognitive development in children8,20–23.
The aims of this paper are three-fold. Firstly, given that childhood is a critical period for brain development, this evidence synthesis aims to analyse the available evidence regarding the possible role of lutein and zeaxanthin for neural development and cognitive function in children. Additionally, the available evidence is explored to identify plausible mechanistic processes through which lutein and zeaxanthin might influence cognitive development. Lastly, given that the circulating levels of lutein and zeaxanthin available for uptake into neural tissue are dependent on their consumption in the diet, the evidence in relation to dietary intake of these phytonutrients in children is also appraised. Such an analysis may be useful as a means to inform dietary recommendations for the optimisation of cognitive development in children.
There is insufficient available evidence to support a full systematic review. An evidence synthesis was, therefore, conducted by performing a comprehensive literature search using the following major databases: PubMed, Scopus, the ISRCTN registry and Cochrane Library with the following search items: “lutein” or “zeaxanthin” or “macular pigment” or “carotenoids" AND “cognitive function” or “memory” or “attention” or “reaction time” or “brain” AND “children” or “childhood” or “infants” or “infancy” or “premature” or “prematurity” or “adolescent”.
The evidence synthesis was confined to peer-reviewed publications. Clinical studies were included as a means to elucidate the evidence that lutein or zeaxanthin status might be associated with or impact cognition in children. Multiple study designs were considered eligible including: meta-analyses, randomised and non-randomised clinical trials, cohort studies and cross-sectional studies involving children (<18 years of age). Non-peer reviewed publications, animal studies, case studies, abstracts, reviews, studies where the full text was not retrievable and studies where the outcomes were deemed not directly related to cognition (e.g. head circumference, broad biomarkers such as activin A) were all considered ineligible for inclusion.
The phenomenon of interest in this analysis was the relationship between dietary intake (including breast milk), serum and/or macular concentrations of lutein and zeaxanthin, and cognition in children.
The final search was conducted in May 2018. No language or date restrictions were used in the electronic searches for trials. This search resulted in a total of 543 potential eligible articles. Two investigators independently reviewed the titles, abstracts and full text of the resulting articles for inclusion. The reference list of each publication meeting the inclusion criteria was examined and ancestry searches were performed on relevant review articles to identify any further studies that were not found using the electronic search. The overall search resulted in the inclusion of six cross-sectional studies, five of which are specific to the effects of lutein or zeaxanthin, while one study reported findings for lutein in combination with the non-carotenoid compound, choline. Data extraction was conducted manually, using the following categories: author, year, methodology, results, conclusions. Qualitative evidence synthesis was then conducted, including assessment of methodological limitations of articles selected for inclusion. Given the qualitative nature of this analysis, no statistical methods were used. Figure 1 below summarises the studies retrieved.
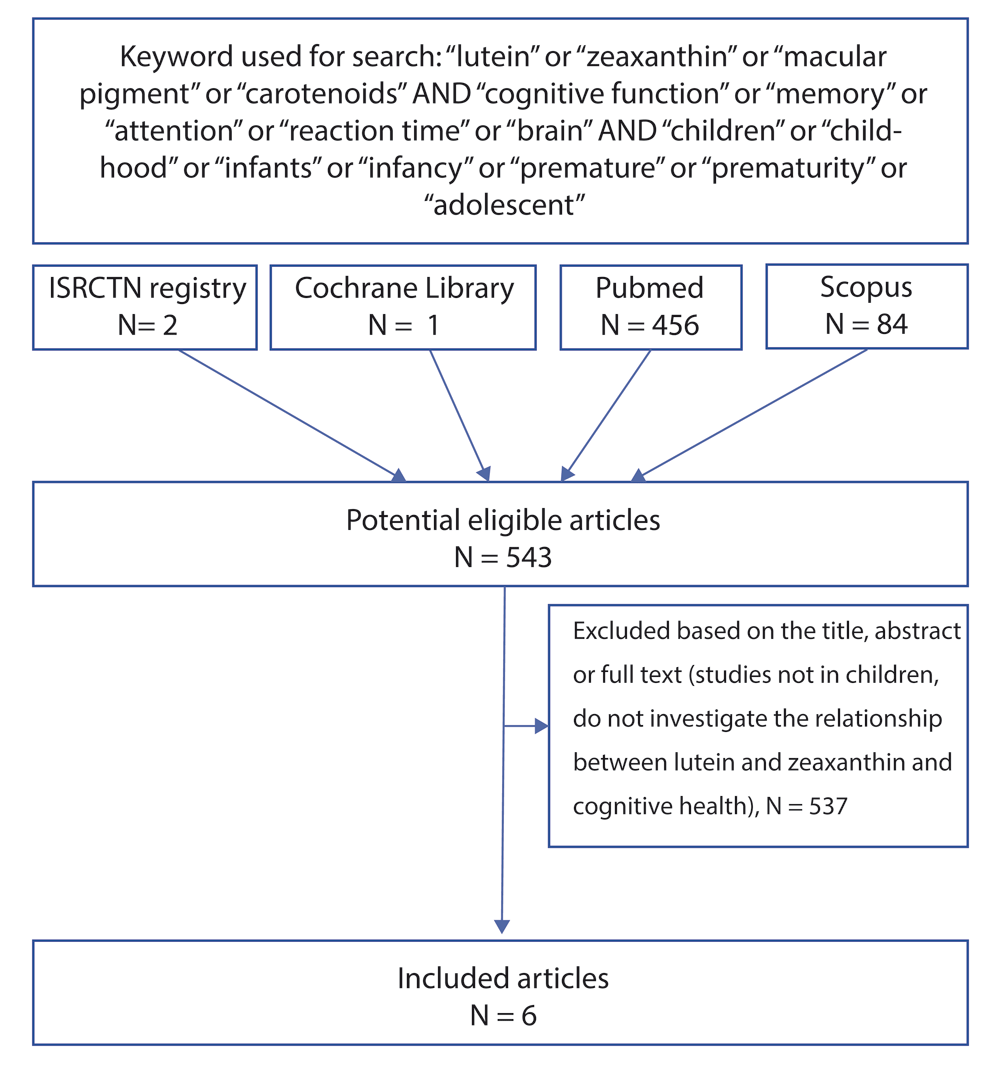
In order to provide mechanistic explanations for any relationships observed between lutein and/or zeaxanthin and cognition in children, additional sources of evidence were explored in the part II of the evidence synthesis including systematic and non-systematic reviews, in-vitro studies, and studies involving adult participants.
The evidence concerning the effect of lutein and zeaxanthin on cognition in children is relatively sparse. Interestingly, however, the evidence that does exist spans the entire range of early childhood development, including from gestation up to the beginning of adolescence (see Table 1).
Although not directly indicative of cognitive function, an investigation of the relationship between arterial cord blood lutein concentrations and activin A, a glycoprotein implicated in the response to acute neuronal damage (e.g. brain injury), provides the earliest indication as to the importance of lutein24. Lutein was shown to correlate positively and significantly with activin A in both male and female populations (p < 0.001 for both). Moreover, both lutein and activin A concentrations showed gestational age-dependent patterns, with peaked concentrations at 33–36 weeks and decreasing from 37 weeks onwards reaching a dip at term. Of note, 33–36 weeks of gestational age is a period of very active central nervous system (CNS) development25,26. The peak of arterial blood lutein concentrations during this period, therefore, suggest the possibility of a specific role for this carotenoid in supporting maturation of the CNS.
Progressing into infancy, a study of 55 six-month-old infants explored the association between human breast milk nutrients (lutein, choline and docosahexaenoic acid (DHA)) and infant recognition memory27. The study revealed that the synergism of higher breast milk lutein with choline was related to better infant recognition memory, which was tested using an event-related potential oddball paradigm. The combined effect was also observed between high choline and DHA. This study was unique in that it evaluated a measure of cognition so early in life. A cautionary interpretation of the findings is necessary, however, as lutein was not analysed as a single ingredient, and because there was a lack of concurrent cognition and milk nutrient measures, whereby human milk was obtained and analysed at three to four months post-partum and infant cognition was evaluated following a further two to three months of development.
Among older children, one study in five-year-olds failed to find a significant association between serum concentrations of lutein and cognitive function28. This study utilised the Kaufman Assessment Battery, an indicator of children’s intelligence quotient (IQ), finding no relationship between the overall score or the subscale scores and lutein. However, the chosen parameters for lutein status were serum lutein and self-reported lutein intake, neither of which are reliable indicators of brain lutein levels29. Furthermore, this study used a population that was particularly well-nourished with an estimated average lutein intake nearly four times higher than that observed in a sample of four to eight year olds in the US National Health and Nutrition Examination Survey (NHANES) survey23. As the population in this study was thereby at low risk for nutrient deficiency, this could have contributed to the lack of detected association.
Academic performance, a global indicator of cognitive function, was also assessed in slightly older children, aged eight to nine years30. Retinal MP levels, which are considered to be reliably associated with lutein concentration in the brain5, were measured using customised heterochromatic flicker photometry (cHFP), a technique shown to provide reliable measures of MP optical density (MPOD) in preadolescent children31. This study demonstrated that MPOD was positively related to academic achievement, mathematics and written language composite standard scores, even after accounting for IQ, sex, aerobic fitness and body composition. Correlations between dietary intake of lutein and zeaxanthin and the same academic parameters were not consistent, which is not surprising given the limitations of serum lutein and zeaxanthin as an indicator of tissue concentrations.
The above findings are supported by the most recent cross-sectional study which explored the association between MPOD and standardised measures of cognitive functioning in 51 preadolescent children aged seven to thirteen years32. Cognition was measured with the Woodcock-Johnson III battery, particularly those tests related to the subcomponents of brief intellectual ability (verbal comprehension, concept formation, and visual matching), verbal ability, cognitive efficiency, processing speed, and executive processes. MPOD was a statistically significant predictor of performance for the brief intellectual ability and for executive processes. Exploratory analysis showed that performance on the spatial relations subtest (a measure of visual-spatial thinking, not included in the calculation of cluster scores) was also statistically significantly related to MPOD. The relationship between MPOD and cognitive efficiency was not significant (r = 0.206, p = 0.074).
In a study by Walk et al. children were asked to perform a modified version of the flanker task, which measures the capacity to selectively attend to a target, while ignoring distractors33. In this study, MPOD was associated with more accurate task performance and lower P3 amplitude of the electroencephalogram (EEG)33. The relationships were more pronounced for trials with high cognitive load. The authors interpret their findings to suggest that children with higher MPOD complete the task more efficiently relative to those with lower MPOD. Therefore, reduced P3 amplitude in participants with higher MPOD could indicate that those kids needed fewer attentional resources to complete the same task compared to the subjects with lower MPOD. The MPOD-dependent variation observed in the behavioural and electrophysiological (EEG) indices elicited during the cognitive control (flanker) task would lend support to such an interpretation.
It appears that MPOD is also positively associated with relational memory in preadolescent children, even when controlling for aerobic fitness and central adiposity34. Interestingly, aerobic fitness was also related to relational memory, but MPOD and aerobic fitness were not associated with each other, suggesting that they may contribute to cognitive performance by different mechanisms. On the other hand, central adiposity was significantly associated with relational memory errors, consistent with previous studies in children and in adults35,36.
In order to describe the possible mechanisms of action through which lutein and zeaxanthin might exert a developmental influence on cognition in children, it is necessary to explore their localisation in the brain, their possible role in early growth and maturation of the brain and the neuro-enhancing influences they may exert as a child develops. In this evidence synthesis, we will also explore a more indirect pathway through which the presence of these carotenoids in high concentrations in the eye might also exert a cognitive influence.
Anatomically and developmentally, the retina is a part of the central nervous system, and both retina and optic nerve originate as outgrowths of the developing brain. The retina and the brain share a number of similarities, including anatomy, functionality, immunology and response to insult37. The retina contains a layer of specialised neurons, the retinal ganglion cells (RGCs), which exhibit properties typical of CNS neurons.
It is well established that lutein and zeaxanthin are present in the eye and in the brain. These two carotenoids have been found in ocular tissues from 18 weeks of pre-natal development, while in the brain they have also been found in preterm infants8,18. The study in preterm infants showed that lutein was a predominant carotenoid in all 5 brain regions analysed (mean concentrations ranging from 40.7 ± 7 pmol/g in hippocampus to 55.52 ± 18.33 pmol/g in the auditory cortex). The concentrations of zeaxanthin ranged from 10.03 ± 2.46 pmol/g in prefrontal cortex to 17.8 ± 3.94 pmol/g in the auditory cortex, considerably lower than lutein.
Lutein was shown to be the predominant carotenoid in the brain of older adults too, although in older adults is accounted for 30% of total carotenoids, compared to 59% of total carotenoids in the infant brain5,8. In older adults, the ratio of brain to ocular lutein (~ 0.4) was slightly higher than brain to retinal zeaxanthin (~ 0.27), but broadly comparable. The ratio in infants has not yet been studied. However, the studies directly relevant to this review focused on either MPOD or breast milk or serum lutein (and not zeaxanthin), so the subsequent analysis will focus on lutein as there is not currently enough evidence to define the possible role of zeaxanthin specifically.
In the infant brain, lutein and zeaxanthin have been identified in prefrontal, frontal, auditory and occipital cortices, and hippocampus8,19. As described earlier, the infant brain appears to be exquisitely sensitive to lutein, capable of accumulating circulating dietary lutein so effectively that it comprises the dominant carotenoid in brain tissue despite limited intake8. That lutein concentration is so much higher in infant relative to adult brain suggests that the effects of lutein are increasingly important in early life. The presence of lutein and zeaxanthin in visual and auditory areas of the brain, as well as in areas associated with executive function and memory, is consistent with associations reported in older children, including possible influences on visual recognition memory in infants27, relational memory34, executive processes and academic performance30,32.
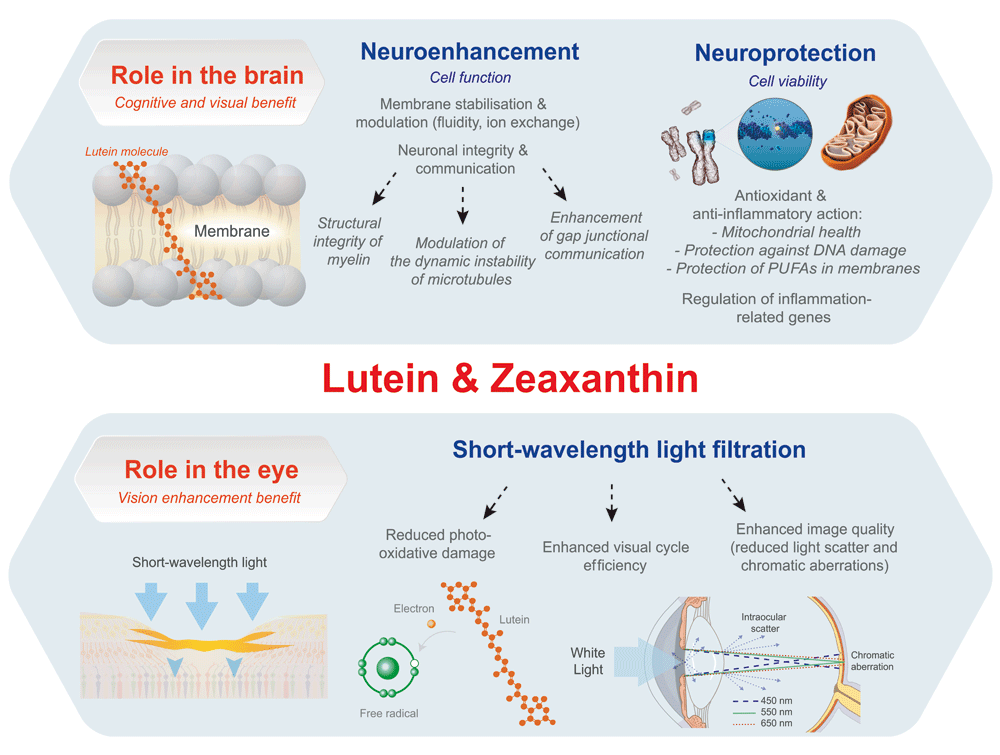
Mechanisms by which lutein and zeaxanthin are believed to protect the retina, such as their antioxidant, anti-inflammatory and neuroprotective action, are likely to apply to the brain as well38. Such neuroprotection is particularly important during infancy when the CNS is developing at a very fast pace. The membranes of the brain and retinal cells contain high quantities of the long-chain omega-3 PUFAs, including docosahexaenoic acid (DHA) - the main n-3 PUFA in the brain, which is essential for maturation of the CNS, but also is easily susceptible to oxidation39. DHA is known to regulate immune function and microglia activation with its derivatives exerting anti-inflammatory and proresolving effects39. Alterations of PUFA metabolism in the brain can be detrimental and lead to neuro-inflammatory events. Lutein, as a potent lipophilic antioxidant that is differentially localised in the membrane domains rich in PUFAs, is well positioned to protect these important lipids40.
A strong relationship has been shown to exist between brain lutein concentration and StARD3, an integral membrane protein which is proposed to be involved in intracellular lipid trafficking. Interestingly, the relationship is strongest during infancy (r = 0.75, P < 0.001), is weaker among older adults (r = 0.51, P < 0.05) and becomes insignificant in centenarians (r = 0.08, P > 0.05), perhaps again suggesting a specific role for lutein in early neural development19. Lutein’s potential role in brain growth and maturation is further supported by findings that it’s content in brain tissue correlates with levels of brain amino acid neurotransmitters (gamma-aminobutyrate, aspartate), neurobiomarkers (activin A) and metabolites of energy pathways (1-octadecanol, phosphate and NADH)41.
Although there is no direct evidence in relation to the infant brain, animal studies have recently provided evidence for a direct effect of carotenoids on the brain through anti-inflammatory and antioxidative mechanisms. Treatment of rats with lutein protected against traumatic brain injury as assessed by a forelimb reach test, and attenuated the increased levels of reactive oxygen species, COX-2, and pro-inflammatory factor NF-kB in the hippocampi of these animals. Concurrently, upregulated levels of Nrf-2, a transcription factor that regulates the expression of antioxidant proteins that protect against oxidative damage triggered by injury and inflammation was observed in the hippocampus42. Another recent report using an animal model, demonstrated that lutein attenuates inflammatory hyperalgesia associated with trigeminal nociceptive neurons in rats through inhibition of COX-243. The direct anti-inflammatory and antioxidant effects of lutein in brain cells has also been explored through its action on microglia. Microglia are the resident immune cells of the brain and when activated develop into either one of two phenotypes, a classically activated pro-inflammatory (M1) phenotype which release inflammatory molecules including cytokines, nitric oxide and other reactive oxygen species that have been shown to have a negative effect on neuronal and cognitive function, or an alternatively activated (M2) phenotype which are primarily involved in neuroprotection by releasing anti-inflammatory cytokines and neurotrophic factors44. M1 primed microglia treated with lutein in vitro resulted in a suppression of the release of the oxidative species inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), nitric oxide (NO) and pro-inflammatory cytokines tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) demonstrating a direct antioxidant and anti-inflammatory effect of lutein in brain cells45. Interestingly, when the effect of combinations of DHA, eicosapentaenoic acid (EPA) and lutein were assessed for their capacity to prevent the release of oxidative and pro-inflammatory mediators from M1 microglia in vitro, results show a synergistic inhibition of NO, prostaglandin E2 (PGE2), iNOS, COX-2, IL-6, and an increase in the release of anti-inflammatory IL-10 from microglia46. These data provide a potential brain-specific cellular mechanistic explanation of recent preliminary clinical evidence demonstrating that a combination of lutein and zeaxanthin with the omega-3 PUFAs, DHA and EPA enhanced memory and mood in a cohort of Alzheimer’s patients47. Thus, as PUFAs are neuroprotective yet vulnerable to oxidation, strategies for dietary interventions that combine PUFAs with the antioxidative properties of carotenoids are worthy of investigation not just for age-related cognitive decline, but for cognitive development in children.
Lutein and zeaxanthin’s protective action on cell viability and their role in ocular and neurological disease such as retinopathy of prematurity (ROP), AMD and Alzheimer’s disease is well documented48–51. Their ability to improve and optimise function of healthy neural tissue, however, suggests that lutein and zeaxanthin may have additional neuro-enhancing effects. Optimal cognitive performance in different age groups and in the absence of disease has been shown to be associated with plasma, retinal and brain concentrations of lutein and zeaxanthin, and to be enhanced by supplementation with these carotenoids12,52–55. The neuro-protection offered by lutein and zeaxanthin is unlikely to account for the observed relationship between these compounds and dynamic brain function in relatively young healthy subjects56,57. Although the exact mechanism behind this observed neuro-enhancement is not clear, there are a few plausible routes whereby lutein and zeaxanthin may improve neural function.
Stabilisation of membranes, modulation of their activity58 and enhancement of intracellular communication are among the suggested mechanisms of action of these carotenoids22,59. Lutein concentration in the infant brain has been demonstrated to correlate with fatty acids and lysophospholipid levels in the frontal cortex and hippocampus, which are known to mediate neuronal signal conduction41. It has also been demonstrated that supplementation with lutein in diabetic mice prevents degradation of the synaptic vesicle protein synaptophysin, a marker of synaptic density60. It is possible that lutein and zeaxanthin increase processing speed through their facilitative effect on gap junctional communication61. Gap junctions are cell-to-cell channels formed by connexin proteins that allow signalling compounds to pass freely between cells62. These channels are believed to be important for the maturation of neural circuitry as they play a role in light processing within the retina63,64.
Unlike nonpolar carotenoids such as β-carotene and lycopene, lutein has polar groups at each end of the molecule which are believed to allow the molecule to span the membrane in a perpendicular or semi-perpendicular orientation to the membrane surface65,66. Together with their high solubility in membranes, this characteristic can positively influence membrane properties including fluidity, ion exchange, oxygen diffusion and membrane stability67, as well as conferring a protective influence in making membranes less sensitive to oxidative damage68.
Lutein and zeaxanthin may also play a role in maintaining cell integrity and plasticity, by binding to tubulin, the major structural protein of microtubules69,70. Being integral to the cytoskeleton, microtubules are important for mechanical, transport, communicative and signalling purposes71. These carotenoids may modulate the dynamic instability of microtubules (the combination of assembling, disassembling, and rapid transitions between the two), and thereby promote cell integrity70.
Although the primary influences of lutein and zeaxanthin on early cognitive development are likely mediated through their selective accumulation directly in brain tissue as outlined above, it is perhaps also worth speculating herein as to the possible benefits that their co-location in the eye might deliver beyond vision and into cognition. The role of MP in the eye is well established. The visual benefits of MP relate to its antioxidant, anti-inflammatory and optical properties, while there is a growing body of evidence that lutein and zeaxanthin may also have a favourable effect on neuronal processing56,72. They may also, however, play an important role in visual development.
In early life, the retina is particularly vulnerable to oxidative stress and inflammation due to immature autoregulation of blood flow in the choroid, increased metabolic activity and increased exposure to short wavelength light due to a highly transparent crystalline lens73. The rationale for the proposed role of lutein and zeaxanthin in visual system development is based on findings that these carotenoids are present in ocular tissue from 18 weeks of pre-natal development18. Additionally, supplementation studies of lutein in infants, particularly those with ROP, have shown possible benefits. Supplementation with lutein in the first hours of life can reduce neonatal oxidative stress74, which is particularly important for new-borns whose antioxidant system has not yet developed. Oxidative stress and resulting inflammation are inextricably linked to the pathogenesis of common diseases of prematurity, such as ROP75. Additional benefits have been shown with lutein supplementation in infants, including reduced inflammation76, and even decreased ROP severity50. An RCT in preterm, very low-birth-weight neonates showed that supplementation with lutein and zeaxanthin resulted in 50% decreased progression rate from early ROP stages to threshold ROP50. MP’s antioxidant, anti-inflammatory and immuno-modulatory properties may, therefore, provide protection to the vulnerable developing retina and potentially brain77–80, and thereby influence visual and possibly cognitive development81.
It is well established that preterm infants more often develop neurological problems than term infants, with the level of impairment ranging from subtle cognitive abnormalities to severe neurological handicap82,83. Even low risk premature infants have lower scores for cognitive development at 3-4 years of age84. Preterm children without measurable neurological damage perform worse on visual perception and visual motor integration tasks, as well as memory, sustained attention and picture vocabulary tests84. Interestingly, MP levels have been shown to relate to such tasks in adults, including visual motor response85 and visual-spatial functioning72. A number of factors could contribute to the development of cognitive deficits in preterm infants, including the same mechanisms of oxidative stress and inflammation involved in ROP pathogenesis. Preterm infants also have brain lutein and zeaxanthin levels that are lower than in the full-term infants8. Lutein and zeaxanthin may, therefore, be uniquely suited to protect the premature brain, as well as retina, from the sudden increase in oxygen levels experienced after birth, compared to in utero.
Although normal vision is not a prerequisite for normal cognitive development, in sighted individuals, vision is one of the main drivers of development through observation and facilitating exploration of the environment. In sighted individuals, vision is generally the dominant sense86,87. Indeed, in sighted individuals, vision-led functions such as distinguishing shapes are important for development of shape bias and associated language learning88. Motion perception is key to categorisation of objects89, which in turn, is linked to semantic memory. Additionally, imitation, which is a fundamental aspect of learning, is grounded in action and movement recognition90,91. In later life, vision appears to support the maintenance of cognitive health, with loss of vision associated with a decline in memory92.
There is some indirect evidence from various sources to support the view that optimised vision (which may be influenced by MP levels) may be a refining factor for optimal neurocognitive development. Firstly, the cortical areas corresponding to the fovea are greatly magnified, with about half of the primary visual cortex devoted to processing information from the macula, reflecting the importance of this area for later information processing by the more anterior portions of the brain93. Moreover, the development of sensory networks, and particularly visual ones, is prioritised over motor networks, suggesting the special role of vision for overall development94.
According to the “effortfulness” hypothesis first proposed by Rabbitt in 1968, an individual’s cognitive processes can be affected by increased effort required when trying to identify degraded sensory input95. It is possible, therefore, that degraded (or non-optimised) visual input may put increased demand on cognitive resources and thereby deprive other cognitive activities. It has also been demonstrated that even a modest reduction in image quality can have a marked effect on the speed of response on cognitive tests in both younger and older adults96,97. In the eye, MP acts as a pre-receptoral optical filter that serves to optimise and refine the visual signal to be delivered to the brain98. How much suboptimal vision and any associated “effortfulness” could affect an individual’s cognitive development long-term remains, however, unclear.
There is evidence that vision impairment can affect language and communication development in children, especially during the early stages99. Moreover, perceptual processes later in life and normal development of the neural architecture appear to depend to a significant degree upon the early visual input received. The deprivation of patterned visual input due to congenital cataracts in new-borns has been shown to result, for example, in permanent deficits in configural face processing, a function mediated by both vision and cortical mechanisms, even after more than nine years’ recovery (cataracts were removed at 2–6 months of age)100.
Whether the visual benefits of lutein and zeaxanthin outlined herein translate into cognitive benefits, however, remains entirely speculative at this time. The benefit of sensory stimulation enhancement, if any, is likely to be small relative to its broader effect inside the brain. There may be other, as yet uncharacterised, roles and mechanisms of action through which carotenoids can exert a positive influence in the developing retina and brain38. An overview of the various mechanisms by which lutein and zeaxanthin might influence cognitive development are described in Figure 2.
In humans, lutein and zeaxanthin cannot be produced endogenously and are, therefore, acquired entirely from dietary sources. The deposition of these carotenoids in the body begins early during pre-natal development, when they are obtained from mother’s blood via placental transport101. Immediately after birth, mother’s milk (or milk formula) becomes the sole source of lutein and zeaxanthin with their respective concentrations in human milk depending on a number of factors including the stage of lactation, maternal intake of these carotenoids102,103, or milk formula constituent ingredients. Once solid foods replace mother’s milk, green leafy vegetables, fruit and eggs become the primary sources of lutein and zeaxanthin, along with other nutrients essential for normal development. If variations in brain and/or ocular levels of lutein and zeaxanthin do indeed influence cognitive development in children, it is important to consider the evidence describing the intake of these carotenoids among children.
The transfer of lutein and zeaxanthin across the placenta is believed to be dependent on their respective concentrations in mother’s plasma, indicating a passive diffusion process and emphasising the importance of mother’s diet during pregnancy and lactation104. While lutein and zeaxanthin are detectable at 18 weeks gestation18,105, the concentrations of lutein and zeaxanthin within the eye increase with later gestational stages. It appears that most lutein deposition occurs during the last trimester8,106, which explains why lutein levels are much higher in neural tissue among term infants when compared to pre-term infants.
Infancy is a critical period of rapid growth and maturation which places high demands on nutritional intake. Human milk is the sole source of nutrients for breast-fed new-born infants until the introduction of solid food, and it provides for all the dietary requirements during the first few months after birth, including lutein and zeaxanthin103,107. In a nine-country survey conducted on breast milk carotenoid composition among 471 women, the overall mean ± SD for breast milk lutein plus zeaxanthin was 25 ± 19 µg/l, but individual country means varied three-fold, from a low of 15 ± 5 µg/l in the U.S. to a high of 44 ± 18 µg/l in Japan. The highest individual lutein concentration measured was 232 µg/l in China and the lowest was 3 µg/l in the U.K, a staggering 77-fold variation102. Interestingly, it appears that breastfeeding and its duration may have effects on cognitive development in children during their first three years of life108. One study has shown that infants who were breastfed for ≥ 9 months had significantly better cognitive development than those who had not been breastfed, although this finding was not explored in relation to nutrient concentrations in breast versus formula milk109.
Although formula milk is designed to contain all the major components of breast milk, breast milk remains the best source of nutrients for infants and contains many additional factors110. Lutein and zeaxanthin have been found in several formula products, with lutein concentrations ranging from 0.7 to 9.7 nmol/g fat and zeaxanthin ranging from 0.1 to 1.2 nmol/g fat in those containing the carotenoids, while none was found in other market-available formulations111.
Human milk samples analysed in this study had comparable levels of lutein and zeaxanthin to the formula milk, with median concentrations of 4.79 nmol/g fat (range 0.42-9.98) and 0.55 nmol/g fat (0.00-1.70) in human milk samples, respectively. However, there is evidence to suggest that lutein in formula milk has lower bioavailability than that in breast milk. One study compared lutein levels in three groups of healthy term formula-fed infants randomised to study formulas containing different concentrations of lutein (from 20 which was considered unfortified to 225 µg /l of lutein) and a breastfed reference group. It appears that the bioavailability difference is such that approximately four times more lutein would be needed for formula-fed infants to achieve similar serum concentrations to breastfed infants112. It is plausible to suggest, therefore, that the relative lack of lutein and zeaxanthin in some formula milk, coupled with the reduced bioavailability of formula derived sources could disadvantage formula-fed infants with respect to carotenoid accumulation in macular and brain tissue.
Given individual and regional differences in breast milk carotenoid concentration, dietary fortification among expectant and lactating mothers merits consideration, and there is some evidence to suggest this can be effective. Supplementing lactating mothers with a formula containing two different concentrations of lutein, in combination with docosahexaenoic acid (DHA) and alpha-tocopherol for 6 weeks resulted in increased total lutein and zeaxanthin in plasma and breast milk compared to placebo113. The infants of mothers assigned to the low- and high-dose lutein supplement also had a significantly greater concentrations of total plasma lutein and zeaxanthin, which correlated significantly with their mother’s breast milk total lutein and zeaxanthin113.
Green leafy vegetables are the major dietary source of lutein and zeaxanthin once a child progresses to solid food. The two carotenoids are found in many of the same foods and most dietary databases include them together114.
The 2015-2020 Dietary Guidelines for Americans recommend specific age-appropriate caloric and vegetable intake levels for children. For children aged 1-3 (caloric intake 1000K), for example, the recommended vegetable intake every week is seven cups of vegetables. This includes 0.5 cup per week of dark green vegetables- the richest sources of lutein and zeaxanthin in the diet115. According to the 2002 Feeding Infants and Toddlers Study (FITS), a national sample of US children 12–24 months old, mean vegetable intake in this age group was just 2.8 cups per week with no documented intake of dark green vegetables116. In another study, only 3.1% of non-Hispanic children consumed dark green vegetables between the ages of 6–11months increasing to 7.5% between the ages of 12–24 months117. This low dietary intake pattern would seem to present the risk of inadequate lutein and zeaxanthin consumption among children.
Some degree of variability has been observed in dietary intake levels among older children. Analyses of a single 24 h diet recall collected in the NHANES in 2003–2004 revealed that the mean (SD) estimated lutein intake among 4–8 year old children was just 311 µg/day (474), with intakes of 335 µg/day (730) for children 9–13 years of age and 432 µg/day (1062) for those 14–18 years of age118. For comparison, in another more recent study of 160 Canadian children (mean age 5.75 years), the mean (SD) lutein intake based on 24 h recall records was somewhat higher, at 1230 (2020) µg/day. This may suggest higher intakes of lutein among children in that study, or possibly increased awareness and availability and hence intake of lutein-rich foods over the decade since the 2003–2004 NHANES survey28. However, the food sources accounting for the higher intake of lutein were not identified. Another study of 13-17 year old Canadian children using one 24-hour recall, reported a mean (SD) lutein intake of 1132 (1888) (5th to 95th percentile range 50-1306) µg/day for 84 males and 1331 (2090) (5th to 95th percentile range 92-1561) µg/day for 94 females119. Of note, the large standard deviation values indicate wide variation and positive skewing in all these studies. The limitations of all methods to quantify dietary intake of specific nutrients do need to be acknowledged. Most importantly, however, dietary intake analysis only provides intake estimates and does not account for the absorption and distribution of carotenoids to target tissues in the body.
Recommended daily/dietary allowances (RDA) are recommendations for the minimum amount of a nutrient that is needed for most individuals to stay healthy. Despite their potential benefits for vision and cognition, lutein and zeaxanthin are not currently considered as essential nutrients, and at present there is no RDA guide for either lutein or zeaxanthin. Intakes of approximately 6mg/day have been associated with a decreased risk of age-related macular degeneration, however, the average American adult consumes only 1-3 mg lutein/day and children appear to consume even less120. For children, the importance of their consumption relates more to the possibilities for optimisation of visual and cognitive development. This is evidenced by the positive association between the quality of diet (particularly increased intake of fruit and vegetables, rich sources of lutein and zeaxanthin) and academic performance as demonstrated in 5th graders121. Although no RDA exists, the evidence cited herein suggests that lutein and zeaxanthin intake levels during the important developmental stages of childhood appears to be highly variable and potentially deficient in many cases throughout all stages of child development.
Of note, nano-encapsulation of carotenoids should be considered as it may enhance the potential of carotenoids for brain function, by increasing stability and bioavailability. The cognitive-enhancing benefits of encapsulated lutein was shown in a study in mice whose performance in a recognition memory task was significantly increased after administration of encapsulated lutein compared to administration of free lutein122.
Obesity represents another potentially important consideration, with childhood obesity becoming increasingly common across the globe which raises concerns relating to changing diet patterns with processed, sugar-laden foods replacing fruit and vegetables. In addition to its detrimental metabolic effects, increased adiposity has been shown to be related to brain health and cognitive function123. Although the consequences of childhood obesity for cognitive development are as yet unknown, it is likely that the effects of excessive body fat and metabolic misbalance on cognitive development are pronounced during childhood, when the brain is still developing and experiences a high degree of plasticity124. Interestingly, adipose tissue is the major storage organ for carotenoids outside the CNS125, and there appears to be an inverse relationship between MPOD and measures of obesity126,127. It has been suggested that body fat acts as a reservoir for lutein and zeaxanthin128, with excessive adiposity and its associated metabolic changes (such as an unfavourable ratio of low- to high-density lipoproteins) potentially contributing to impaired transport and delivery of lutein and zeaxanthin to the eye and the brain129. The possible impact of the obesity epidemic on retinal and brain carotenoid levels and associated visual and cognitive function remain to be seen, and have yet to be explored empirically.
Nutrition during the pre- and early postnatal period of life may have a key role for the prevention of neurodegeneration later in life130. It is possible that simple dietary modification or supplementation with a dietary antioxidant and anti-inflammatory agent such as lutein might exert a tangible impact on early neural development as well as on the delay of age-related cognitive decline22,38,131. The beneficial role of lutein and zeaxanthin for the structural integrity and function of the ageing brain has been demonstrated across numerous studies11. Much less is known about their role during infancy, childhood and pre-adolescence, the critical periods of brain development.
The findings of positive associations between MPOD and cognitive abilities in children provide some initial evidence for the role of lutein and zeaxanthin in cognitive development. Indeed, there are numerous potential mechanisms by which lutein and zeaxanthin may protect and enhance retinal and brain function, and thereby support cognitive development in children, including their antioxidant, anti-inflammatory and neuroprotective properties (see Figure 2). Although not yet supported by intervention trials, these findings highlight the importance of habitual intake of lutein and zeaxanthin in children.
However, the specific linkages between the intake of lutein and zeaxanthin, their biological effects and clinical outcomes in children need to be elucidated to provide the robust evidence base that is needed to enlighten any beneficial effects of lutein and zeaxanthin in early life. Although there is promising preliminary evidence for a positive association between lutein and cognitive performance in childhood, the cross-sectional nature of the few studies available and the lack of RCTs, represents an evidence gap that merits further investigation before any firm conclusions can be drawn. As there is currently no RDA for lutein or zeaxanthin, studies demonstrating its influence on cognition would contribute to the evidence base supporting consideration of lutein and zeaxanthin as important phytonutrients.
This work was supported by the Technological University Dublin “Fiosraigh” Dean of Graduate Research School Award.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: My research area is clinical psychophysics and I have published several studies investigating the potential role of the macular pigment in adults.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Vision health and performance, Nutrition, Vision science.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 26 Apr 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with HRB Open Research
Already registered? Sign in
Submission to HRB Open Research is open to all HRB grantholders or people working on a HRB-funded/co-funded grant on or since 1 January 2017. Sign up for information about developments, publishing and publications from HRB Open Research.
We'll keep you updated on any major new updates to HRB Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)