Keywords
diabetes, retinopathy, screening, microvascular complication, primary care
diabetes, retinopathy, screening, microvascular complication, primary care
Diabetic retinopathy (DR) is the most common microvascular complication of diabetes. DR affects 8.2% of the Irish population over 50 years with type 2 diabetes1 and is the leading causes of blindness among adults of working age2. Regular diabetic retinopathy screening (DRS) leads to the earlier detection of retinopathy and treatment that can prevent or delay the development of diabetes-related blindness. Although DRS is found to be effective, few countries have established a population-based DRS programme. In 2013, the new national programme (Diabetic RetinaScreen) was introduced in Ireland offering free, regular retinopathy screening to people with diabetes.
Ensuring a high uptake of retinopathy screening is challenging3. Prior to the introduction of a national programme, there was variation in attendance at regional screening services in Ireland, with attendance rates ranging from 49–80%4–6. Screening uptake has also been identified as a challenge in countries such as the UK; with attendance rates ranging from 56–90%7. Non-attendance at screening has been identified as a risk factor for poor visual outcomes among those with diabetes8. Factors associated with non-attendance include, younger age9,10, type 1 diabetes9, poor glycaemic control9,11 and lack of awareness of the benefits of DRS or the risk of DR among patients6,12. A recommendation from a healthcare provider6,12 and fear of impaired vision12 have been shown to motivate attendance. Little is known about characteristics which determine the uptake of retinal screening among those with diabetes in the Irish context4,6. The aim of this study was to identify factors associated with participation in a new national retinopathy screening service using data from primary care.
RetinaScreen is a government-funded programme providing free, annual retinal screening, and, if necessary, treatment, to anyone aged 12 years or older with diagnosed diabetes. The programme was commissioned in 2011 and rolled out in 2013 and 201413. The current study was conducted during the initial phase of the programme (2013–2015). In Ireland, there is no national register of people with diabetes. The programme register was populated in 2012 using information from existing national health schemes, specifically pharmacy claims data. GPs or by other healthcare professionals involved in diabetes care can add also add patients to the register by directly contacting RetinaScreen. All those on the register are invited by letter to participate in the programme13, after which they provide consent for the programme to hold and use their contact details and receive an appointment. Once consented they receive an appointment, after which they need to attend. Figure 1 illustrates this process of registration, consenting to and attending the programme. To receive an appointment, people first need to consent to the programme. Once consented they receive an appointment, after which they need to attend.
Members of the target population were people with diabetes aged 18 years and over who were eligible to participate in RetinaScreen during the uptake period of interest, that is, diagnosed with diabetes four months before the end of the uptake period of interest (Practice A: between July 2014 and August 2015; Practice B between November 2013 and December 2014).
Data collection was carried out across two large primary healthcare centres (Practice A and Practice B) located in two different Community Health Organisations in Ireland (Figure 2). Practice A had seven GPs with five practice nurses and approximately 22,000 patients. Practice B had eight GPs with four practice nurses and approximately 20,000 patients.
The two primary care centres used the same computerised IT system, therefore data collection methods described were carried out across both sites. All adults aged ≥18 years with diabetes were identified via the practice database, using the International Classification of Primary Care, Second Edition (ICPC-2) codes for diabetes insulin dependent (T89) and diabetes non-insulin dependent (T90). Duplicates were removed and data were extracted from each individual medical record. Next, the following inclusion criteria were applied: age 18 years and older, community-dwelling, diagnosed with diabetes at least four months before the end of the uptake period of interest (Practice A: before May 2015; Practice B: before September 2014). Exclusion criteria were, a diagnosis of prediabetes or gestational diabetes or diabetes insipidus, no perception of light in both eyes (blindness) as documented in medical records, nursing home residence, visiting patient to the practice.
Data were extracted from eligible individual’s medical record. Each medical record was checked for a RetinaScreen letter (results letter or did not attend letter). RetinaScreen was contacted to establish the status of those who did not have a letter on file. Individuals were then categorised into four groups:
1. Not registered (details were not listed on the RetinaScreen database),
2. Non-consenters (details were listed in the RetinaScreen database but did not respond to the RetinaScreen initial letter asking for individual’s consent to hold and use their contact details),
3. Non-attenders (details were listed on the RetinaScreen database; they responded to the RetinaScreen invitation letter but did not attend screening appointment)
4. Attenders (details were listed on the RetinaScreen database, responded to the RetinaScreen invitation letter and attended appointment).
In each practice, the beginning of the uptake period was defined as the earliest date of the first screening results letter available on file (Figure 2). The end of the uptake period was defined as the last day of data collection; hence the uptake period for each practice was 14 months in duration.
Individual-level characteristics were also extracted from the patient’s medical records and included: date of birth, gender, healthcare cover (medical card/private insurance), diabetes type (type 1/type 2), date of GP diabetes diagnosis (≤2012 vs. >2012) and a previous doctor diagnosis of hypertension. A previous diagnosis of myocardial infarction, congestive cardiac failure, cerebrovascular accident and transient ischaemic attack were defined as macrovascular complications. A previous diagnosis of DR, diabetic neuropathy or diabetic nephropathy were defined as microvascular complications. Each medical record was checked for a results letter from existing retinopathy screening services; attendance at existing retinopathy screening services (for example a private ophthalmologist or previous regional initiative) was categorised into two groups: no evidence of attending existing retinopathy screening services (‘none’) and evidence of attending existing retinopathy screening services (‘previous attendance’). Age (years) was calculated by subtracting year of birth from year of uptake period and was categorised into three age groups (18–39 years; 40–65 years; 65 years and over). Duration of diabetes diagnosis was calculated by subtracting year of GP diabetes diagnosis from year of uptake period and was categorised into three groups (0–4 years; 5–9 years; 10 years and over).
Analysis was carried out in Stata version 13 for windows (StataCorp, College Station, TX). Descriptive statistics were used to summarise characteristics of patients and were stratified according to outcome group. Uptake was calculated as the number of people who participated in the programme (consented and attended) and reported as a proportion of the total who were registered. Group specific differences in categorical variables were analysed using Pearson’s chi-square test. The mean and standard deviation were reported if continuous data conformed to normality and the student t-test was conducted to compare mean differences. If data were skewed, the median with associated lower and upper quartile values was reported and the Kruskal Wallis test was utilised. Associations between predictor variables and programme outcomes were examined with multivariable Poisson regression. Adjusted incident rate ratios (IRR) with 95% CI were generated as a measure of association. Predictor variables were selected based on whether they had been reported in the literature as significant predictors of uptake to diabetic retinopathy screening.
Ethical approval for the study was obtained from the Clinical Research Ethics Committee for the Cork Teaching Hospitals (ECM 4 (o)). Patient consent for the use of their medical records was waived by the ethics committee as no patient records or identifiable data were removed from primary care centres. MT acted as a ‘Data processor’ on behalf of the general practitioner and a ‘Data Protection and Confidentiality Agreement’ was signed by the general practitioner and MT.
A total of 722 people with type 1 and type 2 diabetes were identified during data collection (Figure 3). At the time of data collection, one fifth of patients (n = 141) were not registered with RetinaScreen. A total of 582 people were registered and had been invited to participate in the screening programme. Of these, 66% consented to take part (n = 382), the majority of whom attended screening. Overall, 63% of those who were registered (n = 365), participated; i.e., consented and attended (Figure 3).
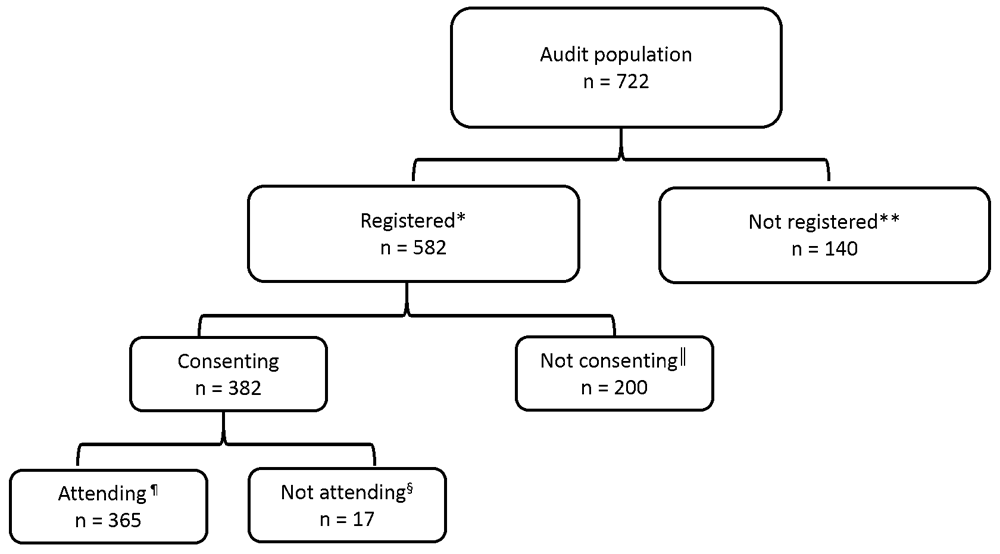
*Registered, details listed on the screening programme database; invitation letter to avail of screening sent. **Not registered, details not listed on the screening programme database. ║Not consenting, did not response to invitation letters; ¶Attending, attended screening appointment. §Not attending, responded to invitation letter but did not attend screening appointment.
Most of the 217 who had not participated in the programme had not consented for the programme to hold and use their details (n = 200, 92%). While the uptake of RetinaScreen was 63% among those who were registered for the programme (n = 365/582), only half (n = 365/722) of the eligible population of people with diabetes had participated in the new national programme at the time of the study.
The characteristics of the 582 people who were registered with RetinaScreen are shown in Table 1. The mean age of patients was 63.0 years (SD 13.8), 61% were male and 91% had type 2 diabetes. Approximately half of the sample had evidence of attending existing retinopathy screening services in their medical record (52%).
| Registered (n = 582) | Consenting (n = 381) | Not consenting (n = 200) | ||
|---|---|---|---|---|
| Variable | Overall (n = 582) | Attending (n = 365) | Not attending (n = 17) | |
| N (%) | N (%) | N (%) | N (%) | |
| Demographics Age years (mean, SD) | 63.0 (13.8) | 63.8 (12.8) | 57.9 (17.6) | 62.1 (15.1) |
| Gender [Male] | 355 (61) | 227 (62) | 6 (35) | 122 (61) |
| Medical card | 406 (70) | 252 (69) | 12 (71) | 142 (71) |
| Diabetes | ||||
| Type 2 | 531 (91) | 341 (93) | 14 (82) | 176 (88) |
| Year of diagnosis ≤2012 | 533 (92) | 337 (92) | 14 (82) | 182 (91) |
| Years since diagnosis (median, IQR) | 8 (5-13) | 8 (5 -13) | 6 (4-10) | 9 (5 -15) |
| Complications Microvascular Macrovascular | 147 (25) 75 (13) | 86 (24) 47 (13) | 5 (29) 2 (12) | 56 (28) 26 (13) |
| Hypertension | 294 (50) | 189 (52) | 7 (41) | 91 (46) |
| Screening history* None Previous attendance | 280 (48) 301 (52) | 214 (59) 151 (41) | 5 (29) 12 (71) | 61 (31) 138 (69) |
Most people who consented to participate in the programme subsequently attended (n = 365/382, 95.6%). Therefore, consent to be invited to participate was the outcome of interest for the regression analysis. Table 2 presents the results from the Poisson regression analyses. Multivariable analysis indicated that people who had previously attended an existing retinopathy screening service (IRR = 0.65 [95% CI 0.5-0.8]; p<0.001) were less likely to consent.
| Variables | Crude IRR (95% CI) (n=582) | p | Adjusted1 IRR (95% CI) (n=582) | p |
|---|---|---|---|---|
| Demographics Age 18–39 years 40–64 years 65+ years Gender Male Female Healthcare cover Medical card Private | 1 (ref) 1.7 (0.9-3) 1.6 (0.8-2.8) 1 (ref) 1.03 (0.82-1.3) 1 (ref) 0.9 (0.8-1.2) | 0.10 0.13 0.68 0.77 | 1 (ref) 1.4 (0.7-2.7) 1.3 (0.6-2.6) 1 (ref) 0.9 (0.8-1.2) 1 (ref) 1.04 (0.8-1.3) | 0.34 0.46 0.69 0.72 |
| Medical factors Diabetes type Type 1 Type 2 Years since diagnosis 0–4 years 5–9 years 10 + years | 1 (ref) 1.4 (0.9-2.1) 1 (ref) 0.9 (0.8-1.3) 0.9 (0.7-1.2) | 0.14 0.89 0.80 | 1 (ref) 1.3 (0.8-1.3) 1 (ref) 0.9 (0.7-1.3) 1.03 (0.8-1.4) | 0.28 0.89 0.81 |
| Attendance to existing retinopathy screening None Existing | 1 (ref) 0.65 (0.5-0.8) | <0.001 | 1 (ref) 0.65 (0.5-0.8) | <0.001 |
This study outlines uptake of DRS among people with diabetes in Ireland during the initial implementation of a new national screening programme. Over the 14-month period the overall uptake (consenting and attending) among people who were registered was 63%. This is similar to the most recent figures (61%) available from RetinaScreen; i.e., people sent a consent letter who attended14, and higher than previously reported in some regional community-based screening initiatives15–17. Consent was the outcome of interest as this is the first point of engagement with the programme before a patient can attend screening. Encouragingly, once consented, most people attended their screening appointment.
National figures indicate that, in the first round of screening (March 2013 to December 2014), of the 134,513 people who were invited to participate (sent a consent letter), 57.1% consented to RetinaScreen13. This is lower than the proportion of people reported in the current study (66%). While previous studies have found factors such as age9,10, type of diabetes and duration9 to be associated with DRS uptake, our analysis only found that previous attendance to an existing retinopathy screening service significantly predicted consenting to take part in the programme. This suggests that people who already are aware of, and familiar with, DRS are more inclined to attend the new national programme. A lack of awareness of DR and the risk has previously been reported as a barrier to attendance6,12,18,19. An Irish study conducted in 2015 which surveyed patients attending general practices and diabetes outpatient clinics about screening behaviours, reported 91% had never previously heard of RetinaScreen20. However, since then the programme has introduced further advertising and may be more familiar to patients.
We found one fifth of patients were not registered with RetinaScreen at the time of the study. The introduction of the Cycle of Care, in 2015, may improve RetinaScreen registration rates for those with a medical card as it provides financial remuneration to General Practitioners (GPs) for providing structured care for people with type 2 diabetes who have a medical card. The structured review visit includes monitoring of key processes of care including screening attendance. In this study, 34% of those not registered would not be eligible for the Cycle of Care. Systems should be put in place to support professionals to register and encourage attendance among all patients. With routine management of type 2 diabetes taking place in the community, primary care professionals are well positioned to promote DRS attendance. A recommendation to attend screening from a primary care professional has been found to motivate attendance18,19,21. In a survey of GPs in Ireland, 56% identified the time required to register patients as a barrier20. Since this study, RetinaScreen have introduced a number of measures to facilitate registration and consent, including an online referral system (2015), and a single step registration and consent form which can be returned by patients directly to RetinaScreen (2019). It is important to recognise that service innovations evolve as they become more embedded in everyday practice. As such, with new implementation strategies Retinascreen may have addressed initial challenges and reasons for non-participation may change over time.
It is important to acknowledge the limitations of this study. First, as mentioned, the study was undertaken during the initial phase of an on-going implementation process. However, estimates from the current study are in line with more recent figures from the programme. Patients may be attending private providers, and we cannot assess this using the current data. Quantitative data were extracted using a standardised extraction template and relevant quality checks were applied to the data. We acknowledge that the completeness and accuracy of our study is dependent on the consistency and timely application of codes in each practice. However, both practices have systems in place to ensure that databases are maintained to a high standard. The type of predictors examined by this study are limited to those available in patient records. Other factors found to be important in previous studies, for example, socio economic status (SES)22–25, self-management, and history of glycaemic control18, could not be included.
We found over one third of people eligible to participate in the free national retinal screening programme, Diabetic RetinaScreen, had not done so. The results suggest DRS attendance could be supported by raising awareness of screening and supporting professionals to register and encourage their patients to attend. Type 2 diabetes, which accounts for about 90% of all cases of diabetes26, is largely managed in primary care, making this a suitable setting in which to introduce strategies to support DRS uptake. Further research is needed to better understand barriers and enablers of DRS uptake in the Irish context, and to determine strategies would effectively target these factors. In Ireland, the population eligible for screening is increasing each year27, therefore implementing effective strategies to maximise uptake of DRS must be a priority from the outset.
Permission was not sought from participating practices or the Clinical Research Ethics Committee to share the data outside of the research team. De-identified data from the current study are available for further (collaborative) research purposes on reasonable request. Available datasets include the audit data. To access the data, please contact the corresponding author (fiona.riordan@ucc.ie) or the Principal Investigator (patricia.kearney@ucc.ie). Researchers must provide a written proposal on how the data will be used in research before access is granted.
Health Research Board Ireland [RL/2013/7].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We would like to acknowledge the contribution of the two primary care practices in which data collection was conducted.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Diabetic retinopathy, screening, ophthalmology, statistics.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Diabetic Retinopathy Screening: A Systematic Review on Patients’ Non-Attendance. International Journal of Environmental Research and Public Health. 2018; 15 (1). Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Diabetes, diabetic retinopathy screening and behavioural medicine.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 13 Dec 19 |
read | |
|
Version 2 (revision) 27 Nov 19 |
read | |
|
Version 1 26 Jul 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with HRB Open Research
Already registered? Sign in
Submission to HRB Open Research is open to all HRB grantholders or people working on a HRB-funded/co-funded grant on or since 1 January 2017. Sign up for information about developments, publishing and publications from HRB Open Research.
We'll keep you updated on any major new updates to HRB Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)