Keywords
cervical screening, HPV, biomarkers
cervical screening, HPV, biomarkers
Cervical cancer is now the fourth most common cancer in women worldwide1. In Ireland, approximately 300 women are diagnosed with cervical cancer each year, with over 90 deaths. In addition, around 6,500 women require treatment for cervical intraepithelial neoplasia (CIN)2,3. Approximately 250,000 to 300,000 women are screened every year in Ireland, making it one of the most important health prevention strategies for our health services.
Infection with human papillomavirus (HPV) is the single most important aetiological factor in the pathogenesis of cervical cancer and pre-cancer. It is now increasingly recognised that HPV is also causally implicated in other cancers, including head and neck, vulval, penile and anal cancer4. Indeed the World Health Organisation (WHO) has recently stated that there is an epidemic of HPV related head and neck cancer, which needs urgent attention.
There are over 150 different types of HPV, 40 of which are found to infect the male and female genital tract. A number of these are known as ‘oncogenic HPV types’ [16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68] based on their potential to cause cervical precancerous abnormalities and cervical cancer5. Of these oncogenic HPV types, HPV 16 and HPV 18 are associated with approximately 70% of all cervical cancer cases6.
Epidemiologically, in relation to HPV there are “three ages of women”:
Age 1: before sexual activity begins (12–14 years), where the risk of acquisition of HPV is negligible. The majority in this age group will be offered prophylactic HPV vaccination.
Age 2: 12–25 years, as sexual activity begins, where girls and young women are exposed to HPV, but the majority of infections are transient and are cleared. A small number of women will develop persistent infection.
Age 3: women 25 years+, where persistent HPV infection is a feature. These women are at risk of developing cervical pre-cancer and cancer.
Infection with HPV is necessary, but not sufficient for the development of cervical cancer. Mild cellular changes and mild dysplasia (CIN 1) may occur after an acute HPV infection, but approximately 90% of these will regress without any treatment7. However, persistent HPV infection may lead to precancerous cellular changes (CIN 2 and CIN 3), a proportion of which will progress, if not treated, to invasive cervical cancer over a period of 10 to 20 years (Figure 1).
Optimal cervical cancer prevention requires both effective HPV vaccination and screening. Because HPV vaccines offered in school-based programmes prevent most but not all high-risk HPV (hrHPV) infections, HPV immunised women should continue to participate in screening.
Population based cervical screening programmes operate in many European countries, including Ireland where a programme, CervicalCheck, has been offering free smear tests to all women aged 25–60 years since September 2008. In addition, HPV vaccination programmes are established in several countries worldwide7 including Ireland, where a national school based HPV immunisation programme began in 2010, using the quadrivalent Gardasil® HPV vaccine, which protects against HPV types 16, 18, 6 and 11. Vaccination is offered to girls (~12yrs) with catch-up immunisation (~18yrs) provided from 2011 onwards. For the first 4 years of the programme, uptake was high (85% among 12 year olds, and 71% for catch-up cohorts)8. However, this has fallen to a low of 50% in 2016/2017 cohorts, with the most recent figures indicating uptake rates of 56%9. This decline in uptake is attributed to un-substantiated claims regarding the safety of the vaccine. The vaccine is considered safe and well tolerated. In March 2015 the US CDC reported that ‘HPV vaccines are safe and effective vaccine’. In November 2015, the European Medicines Agency (EMA) reported on a review of HPV vaccines. This report found no evidence the vaccine was linked to chronic fatigue-like conditions10.
The decline in uptake leads to challenges in relation to the management of cervical disease in this context, as the population becomes stratified into distinct biological and epidemiological risk groups: vaccinated/screened; vaccinated/unscreened; unvaccinated/screened; unvaccinated /unscreened.
Cervical screening aims to detect women with high grade pre-cancerous changes, which can then be treated, reducing the risk of cervical cancer, before cancer actually occurs. Cervical exfoliative cytology is the traditional technique for screening cervical smears to detect and treat cervical pre-cancer. Introduced in the 1940s by Papanicolau, it is considered to be one of the most effective disease prevention strategies ever undertaken.
Like any screening test, it can have false negatives and false positives and its use has to balance sensitivity (the ability of a test to detect disease) and specificity (the likelihood of a positive test identifying underlying disease).
Over the last 5 years, HPV testing has been introduced into the management of cervical pre-cancer. HPV testing is used to triage women with low grade cytological abnormalities, and to manage women following treatment for CIN. It is likely that the use of HPV testing in screening will assume even more importance as increasing proportions of women who have been vaccinated against HPV enter the screening population. The utility of HPV testing has been shown in several international trials with a high negative predictive value (i.e. excluding the likelihood of disease)11–14.
More recently, the use of HPV testing for primary cervical screening has been strongly advocated, as it is significantly more sensitive and has a higher negative predictive value than cytology alone based primary screening.
HPV testing for primary screening is essentially thena “test of risk” rather than a “test of disease”.
In Ireland, a health technology assessment has recently been published byHiQA (Health Informationand Quality Authority, Ireland) at the request of the National Screening Service, to examine the clinical and cost effectiveness of using HPV testing as the primary screening method instead of cytology. This report recommends the introduction of HPV primary based screening for cervical cancer prevention.
However, using HPV as a test of risk is not without its problems. While HPV DNA testing has a very high negative predictive value, it has a low specificity or high incidence of false positives11. Appropriate protocols to stratify HPV positive women are essential to avoid over-referral and over-treatment and thus it will be necessary going forward to develop “tests of disease”, which reflect the cellular response of women’s cells to transforming HPV infection, essentially giving a biological signature of HPV-related disease and disease progression.
HPV DNA testing as a primary screening test is more sensitive than cytology for identifying women who have CIN2+, but the specificity is lower11–14. While high sensitivity is important, many CIN2 and some CIN3 lesions will spontaneously regress as suggested by the natural history of the disease, thus, it is therefore possible that tests with lower sensitivity will still identify those lesions that progress to cancer.
Finding a balance between sensitivity and specificity is hugely important in the context of primary screening to avoid large numbers of unnecessary testing and follow-up of HPV-positive women, which will increase anxiety for women and significantly increase health service-related costs. This could be achieved by avoiding screening in younger women (e.g. <30 years), using more specific HPV tests and using appropriate triage algorithms, based on “tests of disease”.
The majority of evidence from HPV primary screening randomised control trials suggests that reflex cytology is an appropriate option for triage of HPV positive women12,15,16. However, challenges remain on how to manage women who are HPV positive with a negative cytology result. An alternative approach is to triage with some form of secondary biomarker(s). Several biomarker options exist for this, including [1] detection of HPV E6/E7 mRNA, [2] genotyping for HPV16/18, [3] co-expression of p16INK4a/Ki-67 (Figure 2)17,18, [4] detection of a panel of methylation biomarkers (e.g. CADM1, MAL, miR124)19 and [5] using extended panels of biomarkers derived from empirical research(Figure 3).
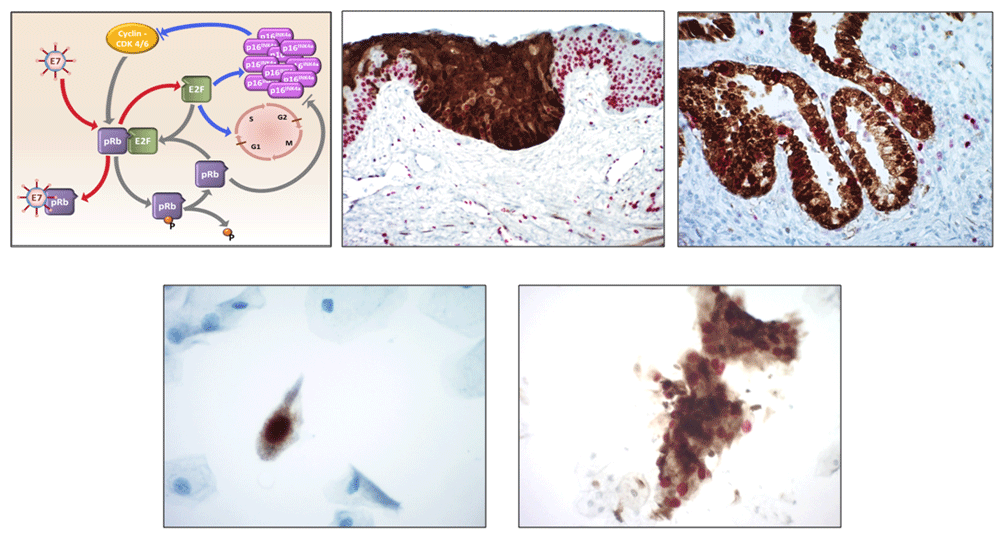
p16 [brown]; Ki-67 [red]. Top Left panel: model of hrHPV E7 protein interaction with cellular transcription factor E2F and its effect on cell division. Top Middle panel: p16/ki-67 staining in CIN 3 [HSIL] (x200). Top Right panel: p16/ki-67 staining in cGIN (x400). Bottom left panel: p16/ki-67 staining in a moderately dyskaryotic [HSIL] squamous epithelial cell (x400). Bottom right panel left: p16/ki-67 staining in a microbiopsy of CIN 3 (x200).
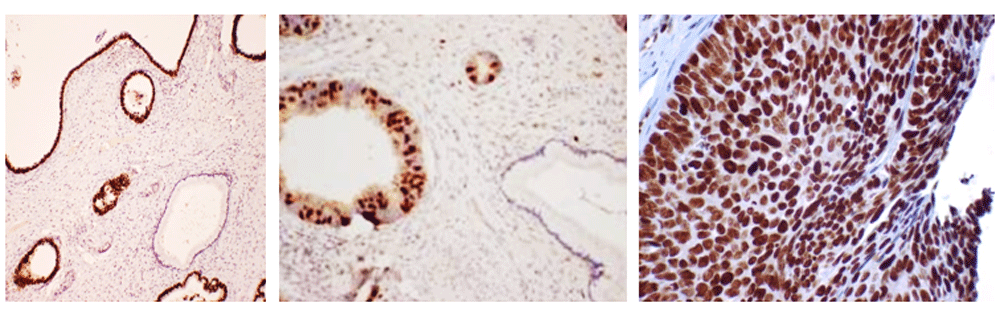
Left panel: Minichromosome maintenance protein 5 [MCM5] in cervical glandular intra-epithelial neoplasia [cGIN] (x200). Middle panel: Geminin expression in cGIN (x200). Right panel: Nuf-2 [replisome associated protein in CIN 3 [HSIL] (x400).
Alternative biomarkers as “tests of disease” have the potential to offer more specific triage of HPV positive women. It is known that HPV subtypes 16 and 18 are associated with over 70% of cervical cancer and consequently induce a higher risk of malignancy. Over expression of viral oncogenes E6 and E7 are necessary for malignant transformation. Detection of these oncogenes by the presence of their mRNA transcripts allows for better distinction between transient HPV infections and those persistent or active transforming infections that are likely to progress to a pre-cancerous or cancerous lesion(s).
The presence of active HPV infections can also be identified through over expression of p16, which plays a major role in cell cycle regulation and Ki-67 a proliferation marker. Over expression of p16 signals E7 mediated deregulation of the cell cycle and thus acts a surrogate marker for active HPV infection (Figure 2).
Recently, methylation of particular genes has been found to be linked to high grade pre-cancer and cervical cancer. Methylation of human genes is strongly associated with CIN and cancer. Several candidate genes are shown to be consistently hyper-methylated in cervical cancer and high-grade CIN, most prominently CADM1, EPB41L3, FAM19A4, MAL, miR124, PAX1 and SOX119–21. These markers show promise as triage markers for managing HPV-positive women, although published studies have been largely cross-sectional with short-term follow-up, in predominantly non-screening populations and conducted on cervical scrapes or self-collected samples21,22. Other novel promising methylation markers include GHSR, SST and ZIC, which are associated with a 3q gain23.
CERVIVA, through its Health Research Board (HRB) funded CARG [CARG29/2012] programme, is evaluating the range of triage options to optimally stratify women with a HPV positive primary screening smear result.
CERVIVA is a multi-institutional, international research ecosystem of excellence working in the area of HPV related diseases. It has spearheaded the development of novel biomarkers as “tests of disease”, based on fundamental biological discovery in relation to HPVs ability to subvert cell cycle machinery in the woman’s cells. It has also developed novel RNA interference and proteosomal degradation drug approaches to alter the fundamental activity of hrHPV transforming genes and proteins.
CERVIVA, in partnership with CervicalCheck, are currently undertaking a HPV primary screening study, which is evaluating and comparing different strategies for the triage of women with a HPV positive primary screening test (www.cerviva.ie). This study, funded by the HRB, isan observational cohort study recruiting>13000 women attending primary care for their routine CervicalCheck smear test (Figure 4).
Women attending their routine CervicalCheck smear tests are invited to participate in the study and give written informed consent. Residual smear samples following routine cytological diagnosis are retained and tested for HPV DNA (Cobas HPV DNA test [Roche]) and HPV mRNA (The Aptima HPV test [Hologic]). Women who test positive for HPV DNA are then tested with a series of triage tests, including cytology, HPV16/18 genotyping, HPV mRNA, p16ink4a/ki67, and a panel of methylation biomarkers. The women will be followed longitudinally through CervicalCheck for up to 10 years through several screening rounds to assess the performance of the different triage approaches for stratifying HPV positive women into different risk categories. This is the first study of its kind internationally that examines all markers in combination, which is embedded within a national screening programme.
Recruitment to the study is now almost completed. Within the population of the first 6,000 women analysed, 15% tested positive for HPV DNA. Overall women under the age of 30 were significantly more likely to test positive for HPV, while those aged 30–39 were at a higher risk of testing positive compared to those aged 50 years and over. The rate of abnormal cytology among the first set of women enrolled is 6.2%.
Of those that tested positive for HPV; 32% were positive for HPV subtypes 16 and 18 the particular subtypes of HPV that are associated with 70% of cervical cancers, and are targeted specifically by the HPV vaccine. In those women that were positive for HPV16/18, 16% had a high grade cytology, compared to only 2% of women who had an infection with another HR HPV subtype. Overall, 43% of those that tested positive for HPV 16 and 18 had an abnormality detected on cytology compared to only 26% of those that tested positive for non-HPV 16/18 HPV subtype.
This early data provides important information to policy makers on the potential impact of changes to cervical screening tests in Ireland. As more information becomes available, it will directly inform decisions around the management of women with a positive screen on HPV-based primary screening tests.
The data will also be helpful in predicting the potential impact HPV vaccination will have on HPV prevalence rates in Ireland. It is likely that significant changes will be implemented to cervical screening as the picture evolves. The challenge will be to build on the achievements of CervicalCheck to date by integrating new and improved tests to prevent more cervical cancers in the future.
Improved detection of CIN2+ and CIN3+, especially in women over 30 years of age.
Long-term possibility of increasing/changing the routine screening interval from 3-yearly to 5- or 6-yearly in women over 30 years of age, thus reducing health economic costs of screening.
Likely to be more effective and efficacious, when prevalence of cervical cancer and its precursors declines in vaccinated populations and the performance of current tests will be challenged.
Appropriate triage methods “tests of disease” will be needed for triage of HPV positive samples to avoid false negatives and false positives.
Long-term follow up of HPV+ women without CIN2+ may be needed.
There remains a risk of 5–15% false negative rates in some women. Therefore careful decisions need to be made around who to screen and when to screen in relation to particular groups.
The views expressed in this article are those of the author(s). Publication in HRB Open Research does not imply endorsement by the Health Research Board of Ireland.
No data is associated with this article.
Health Research Board, Ireland [CARG29/2012; ICE2011/2; ICE2015/1037].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Reagents for the Aptima HPV mRNA test are provided by Hologic Inc. CERVIVA wish to acknowledge the enormous input from CervicalCheck and all of smear takers across Ireland that are helping to recruit women to the study. We wish to also thank the women who have agreed to participate.
Is the rationale for the Open Letter provided in sufficient detail?
Yes
Does the article adequately reference differing views and opinions?
Yes
Are all factual statements correct, and are statements and arguments made adequately supported by citations?
Yes
Is the Open Letter written in accessible language?
Yes
Where applicable, are recommendations and next steps explained clearly for others to follow?
Partly
Competing Interests: I have received speakers’ fee from SPMSD/Merck, served occasionally on the scientific advisory board (expert meeting) of Qiagen, SPMSD/Merck. I have been been co- investigator on a Sanofi Pasteur MSD sponsored trial, of which the study funding went to my Institute. I am part-time director of – and minority stock holder of Self-Screen b.v., a spin off company of VUMC, which holds patents of methylation markers and makes an HPV test and a triage test based on methylation of host cell genes and I have a very small number of Qiagen shares. Until April 2016 I had minority stock of Diassay b.v.
Reviewer Expertise: HPV and cervical cancer, gynaeco-pathology, clinical immunology
Is the rationale for the Open Letter provided in sufficient detail?
Yes
Does the article adequately reference differing views and opinions?
Yes
Are all factual statements correct, and are statements and arguments made adequately supported by citations?
Yes
Is the Open Letter written in accessible language?
Yes
Where applicable, are recommendations and next steps explained clearly for others to follow?
Yes
Competing Interests: Non personal. KCs institution has received consumables and or research funding from the following commercial organisations in the last 3 years: Cepheid, Euroimmun, Hologic, Qiagen, LifeRiver, Genomica, SelfScreen, GeneFirst.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 28 Feb 18 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with HRB Open Research
Already registered? Sign in
Submission to HRB Open Research is open to all HRB grantholders or people working on a HRB-funded/co-funded grant on or since 1 January 2017. Sign up for information about developments, publishing and publications from HRB Open Research.
We'll keep you updated on any major new updates to HRB Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)