Keywords
Patient and public involvement, patient involvement in clinical trials, study within a trial, SWAT, Clinical trial result dissemination, study results, research dissemination, trial results.
This article is included in the HRB-TMRN gateway.
This article is included in the Public and Patient Involvement collection.
Patient and public involvement, patient involvement in clinical trials, study within a trial, SWAT, Clinical trial result dissemination, study results, research dissemination, trial results.
Patient and public involvement (PPI) is increasingly recognised as an essential component of clinical research. In the UK, the national advisory group supporting active public involvement in health services, public health and social care research (INVOLVE) defines PPI as ‘research being carried out ‘with’ or ‘by’ members of the public rather than ‘to’ ‘about’ or ‘for’ them’1. In clinical trials, PPI has been defined as experimenting with participants instead of experimenting on participants2. PPI may occur at any stage during the research process from priority setting and drafting study protocols right through to conducting the study, interpreting the end results and communicating and disseminating research findings3–5. Research funders increasingly expect that PPI is prioritised and resourced within studies. However, there is a lack of evidence on the most effective methods for conducting PPI and its potential impact on the conduct and outcomes of research5,6. In 2001, the need to establish if PPI leads to actual, rather than merely perceived benefits for research processes and output was identified4. Over fifteen years later, this need remains.
In clinical research, the results of clinical trials have not traditionally been shared with clinical trial participants. A recent survey carried out on a large registry of health research participants, found that while 95.6% of respondents said researchers should always or sometimes offer the results to participants, only 33% of respondents actually received the results of studies in which they had participated7. An upcoming European Union Clinical Trial Regulation requires sponsors to provide summary results of clinical trials in a format understandable to laypersons, including participants8. However, there is a lack of evidence on the most appropriate methods of sharing results with participants. Uncertainty persists around what information should be shared, how results should be shared and who should be responsible for sharing the results. Since the findings of clinical research often exist in a complex context of scientific exchange and debate, it is important that the information shared is accessible and relevant to participants9.
The Thyroid Hormone Replacement for Subclinical Hypothyroidism Trial (TRUST) was a multi-centre, double blind, placebo controlled, phase III clinical trial testing the efficacy of thyroxine replacement in subclinical hypothyroidism in older community dwelling adults10. The results of the TRUST trial were published in the New England Journal of Medicine on 3rd of April, 201710. This Study Within A Trial (SWAT) was conducted at the Irish TRUST trial site prior to and after publication of results. The aim of this SWAT was to investigate methods of disseminating trial findings to participants by using a PPI approach to identify, develop and evaluate a patient-preferred method of receiving trial results.
This was a sequential mixed methods study with three phases. In this study, methods were combined for complementarity, where each method addressed a different aspect of the study aim11. The first phase used a qualitative approach to identify and develop a patient-preferred result method, the second phase used a SWAT intervention to compare the dissemination methods and the third phase used a quantitative patient understanding questionnaire to evaluate the patient preferred result method. The full study protocol has been published elsewhere12, but a summary follows here.
The study sites for the TRUST trial were the University of Glasgow, Scotland (lead site); Leiden Academy on Vitality and Ageing, The Netherlands; Leiden University Medical Centre, The Netherlands; University of Berne, Switzerland; and University College Cork, Ireland. A total of 738 participants with subclinical hypothyroidism were recruited to the trial over a three-and-a-half year period from 2013–201710. The trial completed recruitment in November 2016 and the results were published in April 201710.
This SWAT was conducted at the Irish TRUST site. The hub centre for the Irish TRUST site was located at the Mercy University Hospital, Cork where 38 participants were recruited. A further 77 participants were recruited from five satellite sites.
As this SWAT was embedded in an ongoing clinical trial the study sample was determined by the TRUST Thyroid trial. There were 115 TRUST participants recruited in the Irish site, 11 of these participants withdrew over the course of the trial. Our study sample included all remaining TRUST participants (n= 104).
The first phase of the study used a qualitative approach to iteratively identify and develop a patient preferred method of disseminating the results of TRUST trial. This was done in three separate stages: qualitative focus groups, a PPI group and a health literacy review.
Three semi-structured focus groups were conducted with four to eight TRUST trial participants per group. All Cork-based patients (n = 38) were contacted via letter and invited to participate. A €20 shopping voucher was given to all participants to cover travel expenses. Each session was led by trained qualitative researchers (WHS, ER, CH). A topic guide was used to guide the focus groups. The topic guide was reviewed and refined by all members of the SWAT research team (see Supplementary File 1: Focus group topic guide).
The Consensus-Oriented-Decision-Making (CODM) model was used to guide the group to reach a consensus13. The CODM model steps include:
1. Framing the topic
2. Open discussion
3. Identifying underlying concerns
4. Collaborative proposal building
5. Choosing a direction
6. Synthesizing a final proposal
7. Closure
Analysis. Focus group recordings were transcribed verbatim and entered into NVivo Version 11 for data management during thematic analysis. Braun and Clarke guidelines14 for conducting thematic analysis were followed. Initial focus group transcripts were analysed independently by two researchers (ER and AC). Each transcript was read multiple times (data familiarisation) and initial codes were identified. These codes were then used to identify emerging themes. Both researchers discussed emerging themes and conducted further refinement. The refined themes were then discussed and agreed upon with other members of the research team (ER, CH, AC, KMS). Researchers (ER, CH, AC) then used the focus group findings to develop an initial draft of a patient-preferred method for the dissemination of results (see Supplementary File 2: Draft one of patient-preferred result letter).
A PPI group was established to develop and refine the content of the patient preferred method for the dissemination of results. During the focus groups, three TRUST trial participants volunteered to take part in the PPI group. Originally, we intended to conduct these sessions in a group format, due to difficulties with PPI partners’ schedule commitments, one-to-one sessions were conducted. At the one-one session, a researcher (ER) and the PPI partner discussed the layout, content and language of the initial draft of the result method. Researchers and PPI partners worked together to edit different sections of the document. These discussions were not audio recorded but comprehensive field notes were taken by the researcher (ER). These notes were then collated by the researcher and used to further ensure that the results letter reflected PPI partners’ perspectives and preferences.
Researchers then collaborated with the National Adult Literacy Agency (NALA) on the patient preferred result method to ensure the document adhered to national “Plain English” standards. These standards ensure that the information is presented in a way that helps someone understand it the first time they read it15. This review was an iterative process with several drafts exchanged for editing.
At the end of the first phase of the study, a final draft of the patient-preferred result letter was approved by researchers, PPI group and adult literacy experts (see Supplementary File 3: Final draft of patient-preferred result letter).
The second phase of the study used a SWAT intervention to disseminate the results of the TRUST Thyroid Trial to trial participants. This was done using a prospective, randomised, single blind, parallel trial design. It is important to note that when the term randomisation is used, it refers to the allocation of patients to intervention/control within the SWAT and not the TRUST Thyroid trial. Irish TRUST participants were randomised to intervention or control groups using an online random number generator. The intervention group received the patient preferred letter format (see Supplementary File 3: Final version patient-preferred results letter) and the comparison group received a copy of the TRUST results press release, which was made available by the lead study site on the TRUST Thyroid Trial Website (see Supplementary File 4: Standard results letter). Participants were blinded to their intervention group. One member of the research team was un-blinded in order to perform the randomisation and distribute the results of the trial.
The third phase of the study used a quantitative patient understanding questionnaire to evaluate the patient preferred result method. The questionnaire was developed in consultation with experts in the area of subclinical hypothyroidism and scale (questionnaire) development (PK and KMS). The early development of the questionnaire was guided by a consultation document, which accompanies the EU Clinical Trials Regulation No 536/201416. This document highlights the information that trial participants should understand after reading their trial summary. However, initial questionnaire items were modified to allow for psychometric testing. The final questionnaire contained 12 questions; six items were measured on a five point LIKERT scale, there were four multiple-choice questions and two vignettes. The first six items measured patients’ perceived understanding of results, the four multiple choice measured patients’ actual understanding of results by requiring them to select the correct answer. To further test participants’ understanding of the trial results, two vignettes describing two typical patient case studies of older adults with subclinical hypothyroidism were provided with a question on whether a doctor should prescribe thyroxine for the hypothetical patient described. The questionnaire was reviewed by the PPI group to assess content and face validity. It then underwent further review by NALA to ensure adherence to the national ‘Plain English’ standard. The final version of the questionnaire can be seen in Supplementary File 5: Patient understanding questionnaire.
The questionnaire was sent to all Irish TRUST participants (intervention and control group) one week after they received the results of the trial. A reminder questionnaire was sent to non-responders 3 weeks later.
Analysis. The primary outcome was the difference in levels of patient understanding between the intervention and comparison groups. This measured the impact of PPI on patient understanding of end of trial results. The psychometric properties and construct validity of the questionnaire were examined with exploratory factor analysis. Principal component analysis (PCA) was conducted on the six LIKERT scale items. Internal consistency of the questionnaire was investigated using Cronbach's alpha coefficient. Completed questionnaires were entered into SPSS software (version 24) and analysed using descriptive and inferential (Chi-square test and Fishers Exact) statistics. The researcher carrying out data input and analysis was blinded to the participants' allocation status.
The lead researcher (ER) kept a detailed account of all direct costs associated with conducting PPI for the purpose of this study. These costs included researcher salary, travel and expenses for PPI participants, and printing and postage costs.
This paper has been written in adherence to the Guidance for Reporting Involvement of Patients and Public 2 (GRIPP 2)17. The GRIPP 2 checklist is a tool, developed to improve the reporting of patient and public involvement in research and guide the development of a transparent, consistent and high-quality PPI evidence base. The Good Reporting of a Mixed Methods Study (GRAMMS) framework was also used to inform the reporting of the findings18.
Characteristics of the trial participants stratified by participation in the different stages of the study are presented in Table 1.
Three focus groups were held with 19 out of 38 participants accepting an invitation to join. Participants who attended the focus groups were similar in age, gender, education level to those who did not attend.
Focus group findings indicate that participants want to receive the results of the trial in which they are taking part. Three main themes emerged in relation to participants’ perspectives of and preferences for receiving trial results: ‘acknowledgement of individual contribution’, ‘contributing for a collective benefit’ and ‘receiving accessible and easy to understand results’.
Many participants reported feeling they had made an individual contribution to the trial in terms of their time and personal information while attending the trial study visits. As such, participants felt that receiving the results of the trial would provide an acknowledgement of this individual contribution:
While participants spoke about making an individual contribution to the trial, they felt that their involvement contributed to a collective benefit or greater good. Participants reported that receiving the results of the trial would help them to feel that they had contributed to this greater good:
‘I’m not really interested in my own personal results but as the results of the scheme as a whole. You know the idea is, does the study help or hinder old people and that’s what I want to know’ (FG2 P1)
This feeling of contributing for a collective benefit was further reinforced when participants discussed their desire to understand how the results of the trial will be implemented by medical experts and ultimately how it will affect others who have the condition:
Participants expressed a clear need to receive the results of the trial in an accessible and easy to understand way. This preference applied to not just the preferred result method but also to the format, language and content of that result method.
The majority of participants said they would like to receive the results in a letter format posted to them directly from the TRUST trial. Participants felt that this method would be accessible to them as they could read the results ‘in text’ (FG3 P4) and keep a ‘hardcopy’ (FG P1). While participants wanted an official statement of the results in a letter format, they also felt it was important to add a personal element to the letter. They suggested this could be done by offering participants a phone number that they could call if they wished to discuss any further issues or concerns with the TRUST study team:
‘Could you attach a helpline on to it? If you know, somebody had some kind of serious medical question or that they thought was a bit personal element or whatever. That they’d like to talk to a medical person or whatever. Instead of just talking to your GP, maybe that would add another dimension of care around the TRUST’ (FG2 P3)
Participants agreed that the format, content and language of the results letter needed to be easy to read and understand. All participants wanted the letter to be no longer than 2–3 pages and presented in a question and answer format. Participants believed the content of the results letter should include ‘pertinent information’ (FG1 P7) relating to the trial itself, the study drug (including side effects) and the results of the trial. They stressed the importance that this information needed to be informed by medical experts and ‘from a good authoritative source’ (FG2 P2) but it should be presented to them in a language that fits their current context and could be easily understood by those who do not have scientific or medical backgrounds.
‘Just in ordinary language that we can understand ourselves, you know that we don’t want big and long explanation or that, just that we can pick it up straight away that it’s without any huge number of pages. Just the bare, to me anyway, answers to the questions.’ (FG3- P2)
It was evident from the focus groups that participants want to receive the results of the trial both to acknowledge their individual contribution to the trial and also help them to feel that they had contributed to a greater good. Participants expressed a clear preference to receive the results in an accessible and easy to understand way. These results were used by the researcher (ER) to develop an initial draft of the results letter (see Supplementary File 2: Draft one patient-preferred result letter).
The initial draft of the results letter was then further iteratively developed by the PPI group. In total, four participants took part in these sessions (three trial participants and one older adult). Each session contained an open discussion between the researcher (ER) and PPI partners on the layout, content and language of the document. Researchers and PPI partners worked together to write, re-write, edit and change different sections of the document.
This draft was then iteratively reviewed and approved by health literacy experts from the NALA (see Supplementary File 3: Final version patient-preferred results letter).
There were a total of 101 Irish TRUST participants randomised to the SWAT intervention. Trial participants from the PPI group (n = 3) were excluded from randomisation as they reviewed the content of the intervention method prior to the intervention. The intervention group (n=51) received the patient preferred letter format (see Supplementary File 3: Final version patient-preferred results letter) and the comparison group (n=50) received a copy of the TRUST results press release, which was made available by the lead study site on the TRUST Thyroid Trial Website (see Supplementary File 4: Standard results letter).
The overall response rate for the patient understanding questionnaire was 68% (69/101). The response rate for the intervention group was 74% (38/51) and the response rate for the control group was 62% (31/50). There were no significant differences in age, gender and education between those who returned the questionnaire and those who did not.
Post hoc power calculations showed that the study was underpowered to detect an effect. Power for each of the patient understanding components ranged from .01 to. 58.
Table 2 below shows the results of patients’ perceived understanding of the purpose and context of the TRUST Thyroid Trial. Due to low participant numbers across the five Likert responses, the questionnaire response bands have been contracted from ‘Strongly Agree’ and ‘Agree’ to ‘Yes, ‘Strongly Disagree’ and ‘Disagree’ to ‘No’ and ‘Neither agree nor disagree’ to ‘Neutral’. The results show that patients’ perceptions of understanding are similar between the intervention and control groups. Subgroup analysis showed patient’s understanding was not significantly impacted by age, gender or educational level.
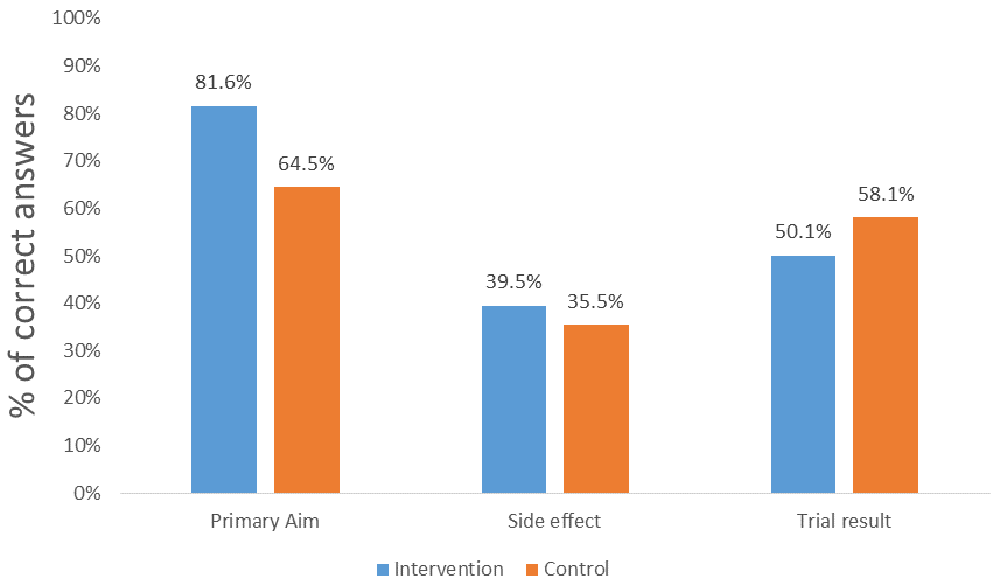
Figure 1 shows patients’ actual understanding of the primary aim, side effect and results of the TRUST Thyroid Trial. Almost 82% (n=31) of the intervention group and 65% (n=20) of the control group correctly understood the primary aim of the TRUST trial (p=0.108). Almost 40% (n=15) of the intervention group and 36% (n=9) of the control group correctly understood the associated side effects of the active drug (p=0.734). In total 50% of the intervention group (n=19) and 58% of the control group correctly understood the results of the trial (p=0.504). There were no differences in patient understanding of trial results between the intervention and comparison groups.
In terms of patient understanding of hypothetical patient case studies, 43% (n=13) of the intervention group gave the correct answer to Vignette A; this was lower than the control group (62.1%, n=18, p=0.15). In total 77% (n=23) of the intervention group gave the correct answer to Vignette B, this was higher than the control group (66%, n=19, p=0.344).
An exploratory principal components analysis (PCA) was conducted on the patient understanding questionnaire to determine its usefulness as a measure of perceived understanding. The Kaiser-Meyer-Olkin (KMO) measure verified the sampling adequacy for the analysis, KMO= .83. Bartlett’s test of sphericity indicated that the correlation matrix was significantly different from an identity matrix, X2 (.852) = 283.92, p<.001. An examination of eigenvalues greater than Kaiser’s criterion of one, suggested the extraction of one factor; this was supported by inspection of Cattell’s scree plot. An examination of the constituent items for this factor structure also indicated that items loaded most highly on a single factor. This single factor represents a measure of perceived understanding of trial results. PCA was then conducted using an oblique (direct oblimin) rotation, specifying the extraction of one factor. This model explained a combined 69.58% of the variance in patients understanding of the TRUST thyroid trial.
The total cost of this study amounted to €8,049 (see Supplementary File 6: Costs of conducting PPI). This includes researcher salary for three months, expenses for PPI partners, and printing and postage costs.
The involvement of clinical trial participants in this study offered insightful perspectives on the information needs of the study population in terms of receiving end of trial results. Study findings show that trial participants want to receive the results of the clinical trial in which they had participated. This is supported by much of the available literature on patients’ preferences of receiving results, with up to 90% of participants in previous studies reporting a desire to receive results19.
Unsurprisingly, findings also show that participants want to receive results that are accessible and easy to understand. In this study, the preferred format of receiving results was a letter posted to them directly from the TRUST trial. This preference is also consistent with the literature on patient preferences of receiving results, with prior literature indicating that 80% of trial participants preferred to receive the results by post20. The patient preferred method identified was feasible for researchers to develop with significant involvement from trial participants and adult literacy experts.
Previous studies exploring participants’ reactions found that sharing trial results with participants can cause some negative reactions such as anxiety, anger, guilt, upset and confusion21–23. As far as researchers in this study are aware, providing results did not cause any negative reactions. Both result methods contained the telephone number, email address and postal address of the research team and participants were urged to contact should they have any questions or concerns relating to the study. The research team did not receive any queries.
While PPI is increasingly recognised as an important element of clinical research, evidence on optimal methods and potential impact is lacking5,6. Previous research conducted on the impact of PPI has largely focused on the experiences of participants and researchers24 and on the research process in broad terms l25. In this study, our primary outcome was specific: a quantitative measure of patient understanding of trial results between those who received the patient-preferred method and the standard method. To our knowledge there has been no previous research conducted on the impact of PPI on patient understanding of trial results.
Previous systematic reviews highlight the lack of evidence on economic analysis of PPI and call for researchers to consider the costs of its implementation25,26. As discussed previously research funders are increasingly demanding that PPI be carried out in research. However, the costs of PPI are often underestimated and can cause a significant financial burden on research project budgets25–28. If these costs are not correctly estimated during the initial stages of developing research proposals, they may also cause a financial burden on PPI partners. As the primary focus of this study was to carry out PPI, researchers carefully considered the costs at grant proposal stage and wrote these costs into the budget.
Participants in this study were not paid for their time but were provided with a €20 voucher to cover travel expenses. When PPI is not the primary focus of a study, researchers do not consider the cost implications at the beginning of the study and are often tied with limited resources to carry out PPI27–29. Existing research suggests that negotiating the mechanisms of paying participants can be difficult. One study reported that in order for participants to be remunerated for their efforts, they needed to be registered as employees, a process that incurred much paperwork and time delays27. This study outlines the cost of conducting PPI and includes a full breakdown of costs (see Supplementary File 6: Costs of conducting PPI). This breakdown provides a template to other researchers who plan to carry out PPI as part of their research.
While this study has provided important insights into patients’ preferences of receiving trial results, it is not without limitations. Firstly, the results of the patient understanding questionnaire show that the levels of patient understanding were similar between the two groups. However, this study was underpowered to detect an effect. As this was a Study Within A Trial (SWAT), the power was limited by the sample size that was available to us from the trial (n=115). Furthermore, validation of the patient understanding questionnaire was limited by the sample size in this study. While validation of the questionnaire was limited, exploratory factor analysis provided some evidence that the questionnaire is a useful tool for measuring patient understanding of trial results. The developed questionnaire can be tailored for use in other trials in future examinations of patients understanding of trial results. This would provide insight into patient understanding and provide further validation data.
Secondly, all trial participants were aged 65 and over. The layout, format and language of this patient preferred method which was identified and developed may only be relevant for this study population. Alternative study populations may prefer to receive the results via email, online or in person from a member of the study team9. The evidence on patient preferences of receiving trial results is limited, therefore further research is needed to explore patient preferences of receiving trial results amongst different study populations.
Thirdly, existing PPI literature states that ‘to understand the research needs and challenges, PPI has to engage people who are able to offer perspectives from the study population’3. However, it is important to note that the PPI partners in this study were already active members of the research community as they had taken part in the TRUST trial and had agreed for long-term follow up. This has an important implication for their reporting of understanding the results of the trial. They may be more inclined to rate their understanding as high because of their investment in the trial30, thus potentially minimising differences between the intervention and control conditions and minimising inferences that can be drawn about the intervention.
It is also important to point out that the control group in this study received a copy of the trial results in a press release format. Most trial participants do not receive this. While this control method was a step further than normal procedure, the researchers in this study felt this was a necessary step to adhere to ethical requirements and effectively compare the intervention and control methods. Given the fact that press releases are written by public relations professionals with a view to communicating effectively and efficiently, this again may have potentially minimised differences between the intervention and control conditions.
Finally, as PPI is a relatively new concept, the majority of the literature has only been published in the last 12 months. There is currently no gold standard or comprehensive guidelines for researchers to follow20. Thornton2 suggests that in order for PPI to develop it is important to record its social and cultural history by collecting comprehensive databases and undertaking ongoing reviews of the impact of PPI. This paper along with the study protocol have been written in adherence with the Guidelines for Reporting Involvement of Patients and the Public17, thus providing templates for involving patients and the public in clinical trial design and development. While this study provides a record of the process of conducting PPI as part of a SWAT, future research is needed to further develop PPI in clinical trial settings.
Patient and Public Involvement (PPI) is advocated for every step of the trial process. We have demonstrated that it is feasible to do this with regard to the dissemination of results. Sharing clinical trial results with participants in a format understandable to laypersons will soon be a legal requirement8. However, there is a significant lack of evidence as to the most appropriate methods of sharing results with participants. The study identified and developed a patient-preferred method of receiving clinical trial results for older adults over 65 years. Although, in this study PPI did not influence patients’ final understanding of result, it provides a record of the process of conducting PPI within the clinical trial setting.
The research was approved in Ireland by the Clinical Research Ethics Committee of the Cork Teaching Hospitals, UCC, Ref ECM 4 (t).
All participants provided signed informed consent to take part in the study.
The raw data from this study cannot be sufficiently de-identified, and therefore are not publicly available. However, the data from the current study are available for further (collaborative) research purposes on reasonable request. Available datasets include transcripts from focus groups, field notes from PPI sessions and responses from the patient understanding questionnaire. To access the data, please contact the corresponding author (emmy.racine@ucc.ie) or the Principal Investigator (patricia.kearney@ucc.ie). Researchers must provide a written proposal on how the data will be used in research before access is granted.
Health Research Board –Trials Methodology Research Network (HRB-TMRN) under the Study Within A Trial (SWAT) funding programme (TMRN-2014-1).
The TRUST Trial was supported by a research grant (grant agreement number 278148) from the European Union FP7-HEALTH-2011 program and by grants from the Swiss National Science Foundation (SNSF 320030-150025 and 320030-172676, to Dr. Rodondi) and the Swiss Heart Foundation (to Dr. Rodondi) and Velux Stiftung (974a, to Dr. Rodondi). Christine Baumgartner was supported by the Gottfried and Julia Bangerter-Rhyner Foundation. Study medication was provided by Merck Darmstadt.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
All supplementary files are contained in one PDF document.
Click here to access the data.
Supplementary File 1: Focus group topic guide
Supplementary File 2: Draft One of Patient-Preferred Result Letter
Supplementary File 3: Final Draft of Patient-Preferred Result Letter
Supplementary File 4: Standard Results Letter
Supplementary File 5: Patient Understanding Questionnaire
Supplementary File 6: Costs of Conducting PPI
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: HB is currently working on a related project, Reporting of clinical trial results appropriately to participants. ST has no conflicts.
Reviewer Expertise: Trial methodology, feedback provision to trial participants
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 22 Mar 19 |
read | |
|
Version 1 11 Apr 18 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with HRB Open Research
Already registered? Sign in
Submission to HRB Open Research is open to all HRB grantholders or people working on a HRB-funded/co-funded grant on or since 1 January 2017. Sign up for information about developments, publishing and publications from HRB Open Research.
We'll keep you updated on any major new updates to HRB Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)