Keywords
Cancer, Primary care, Referral pathways, Early cancer detection, Stakeholders, Qualitative research
This article is included in the HRB Primary Care CTNI gateway.
Early cancer detection improves survival, but many cancers present initially with non-specific symptoms of cancer (NSSoC) such as weight loss, fatigue, or unexplained pain. These presentations are associated with longer diagnostic intervals and a later stage at diagnosis. Several countries have implemented diagnostic pathways for NSSoC, yet Ireland has no national model.
To explore the perspectives of patients, clinicians, managers, and policymakers to inform the design of an NSSoC referral pathway for Irish primary care.
Qualitative study using semi-structured interviews with general practitioners, hospital specialists, service managers, policymakers, advocacy representatives, and patients. Purposive sampling will achieve diversity by role, experience, and geography. Data will be collected via Microsoft Teams, transcribed verbatim, anonymised, and analysed using Framework Analysis structured by the Consolidated Framework for Implementation Research (CFIR). Findings will be mapped to the Engineering Better Care (EBC) framework to generate system-level design recommendations.
Identification of barriers, facilitators, and contextual determinants of an NSSoC pathway; practical, system-aware design principles for a patient-centred referral model suitable for the Irish context; and implementation considerations for policy and service planning.
Cancer, Primary care, Referral pathways, Early cancer detection, Stakeholders, Qualitative research
Cancer remains a leading cause of mortality and morbidity globally and in Ireland1. More than 40,000 new malignancies are diagnosed annually in Ireland, and—if current trends persist—incidence is projected to double by 20452. Although therapeutic advances continue to improve survival, the stage at diagnosis is the strongest prognostic determinant. For several common solid tumours, including colorectal and lung cancer, five-year survival exceeds 80% when diagnosed at stage I but falls below 20% at stage IV2,3. Accordingly, earlier diagnosis is prioritised within Irish and international cancer control strategies as a means of reducing mortality and limiting treatment-related morbidity4,5.
In Ireland’s gatekeeping system, general practitioners (GPs) are usually the first point of contact for patients with new or concerning symptoms and control access to most specialist investigations and referrals. Primary care is the most effective setting for early cancer detection, given that most of the population interacts with GPs annually. In 2024, 79% of the Irish population visited a GP at least once, averaging 4.4 visits per person6. In contrast, fewer individuals consulted hospital specialists (36%), attended emergency departments (16%), or used out-of-hours GP services (9%) in 20237. These patterns highlight the extensive scope of primary care and its vital function in early symptom recognition, investigation, and prompt diagnosis.
Structured “fast-track” or “urgent suspected cancer” pathways for site-specific red-flag symptoms—such as a palpable breast mass —have improved diagnostic intervals and stage distribution at presentation4. However, these condition-specific pathways are not well suited to people whose cancers present initially with non-specific or low-risk, but not no-risk, symptoms, where the pre-test probability is modest and the likely tumour site is unclear. In the absence of a dedicated pathway, GPs with a high level of concern or clinical suspicion often rely on urgent care pathways, such as ‘Acute Medical Units’, or on community imaging direct access schemes, where they independently coordinate the diagnostic work-up and resultant findings.
Non-specific symptoms of cancer—including unintentional weight loss, persistent fatigue, unexplained pain—carry low individual positive-predictive value for any single tumour site yet collectively account for approximately one in four cancer presentations1,2. Extensive primary-care cohort studies show that patients with NSSoC experience substantially more pre-referral consultations, longer primary-to-diagnosis intervals, and a two-fold higher likelihood of late-stage disease compared with patients who present with classical alarm features3,4. For GPs, decisions about escalation and investigation demand careful balancing of competing risks: avoiding missed cancers while minimising over-investigation and judiciously stewarding finite diagnostic capacity4. For patients, repeated consultations without diagnostic resolution can erode trust, exacerbate anxiety, and defer treatment initiation.
The clinical and system-level sequelae of late-stage diagnosis are well recognised. Advanced disease entails more intensive, toxic and costly therapies, poorer quality of life and markedly worse survival outcomes8,9. Health-system impacts include increased treatment costs, pressure on hospital capacity, and widening socioeconomic disparities, given that diagnostic delays disproportionately affect disadvantaged populations8,9. The psychological burden is also considerable; qualitative research describes frustration, self-doubt and stigma among individuals whose symptoms were initially attributed to benign causes2. These consequences strengthen the case for pathways that can safely accelerate diagnostic assessment for NSSoC.
Several European health systems have introduced dedicated models to address NSSoC. In Denmark, Diagnostic Centres provide rapid access to targeted laboratory panels, cross-sectional imaging and multidisciplinary review for patients with serious but non-specific symptoms; evaluations report short median times to diagnosis and high clinician acceptability10. In England, Rapid Diagnostic Centres integrate primary-care referral templates, standardised triage algorithms and one-stop investigative workups11. Sweden, Norway and the Netherlands have implemented comparable approaches, each adapted to local service configurations12. Although programme designs vary, common features include clear primary-care referral criteria based on symptom clusters or risk scores, direct access to cross-sectional imaging and key blood tests, streamlined multidisciplinary assessment, and active navigational support to guide patients through the diagnostic pathway. Early evidence suggests reductions in diagnostic intervals, improved patient experience and potential cost-effectiveness once downstream treatment savings are considered13. However, most evaluations are observational, and the extent to which outcomes depend on contextual factors—such as funding mechanisms, workforce availability and rurality—remains insufficiently understood.
Despite strategic commitments to earlier diagnosis, Ireland currently lacks a nationally coordinated referral pathway for NSSoC5. Access to diagnostics is uneven, with substantial inter-county variation in waiting times for CT, MRI and endoscopy13. GPs report uncertainty about thresholds for requesting imaging, inconsistent feedback on referrals and limited administrative support for tracking serial investigations14. Inconsistent management of incidental findings on cross-sectional imaging can lead to both overuse of resources and delayed diagnosis. In the absence of explicit referral criteria and protected diagnostic capacity, patients with NSSoC often navigate a fragmented system, oscillating between primary and secondary care before definitive investigation. This fragmentation risks prolonging diagnostic intervals, amplifying inequities—particularly for individuals in rural areas or with constrained resources—and increasing pressure on acute services. Addressing these shortcomings requires an approach that is context-appropriate and feasible within existing capacity constraints.
Introducing an NSSoC pathway constitutes a complex intervention spanning multiple professional groups, organisations and policy domains. The Medical Research Council’s guidance for complex interventions recommends preliminary qualitative work to map needs, barriers and enablers prior to pathway prototyping and evaluation15. In Ireland, stakeholder engagement is essential for clinical feasibility, operational sustainability and equity. Clinicians must trust the referral criteria and receive timely, bidirectional communication; managers and policymakers must balance additional diagnostic demand against finite imaging, pathology and workforce resources; and patients and carers can overcome access barriers linked to geography, socioeconomic position, language and health literacy, ensuring that implementation does not inadvertently widen disparities.
A theory-informed approach can help organise this complexity. The Consolidated Framework for Implementation Research (CFIR) offers a comprehensive taxonomy to identify multilevel determinants related to the intervention, organisational context, individual actors and implementation processes16. The Engineering Better Care (EBC) framework complements CFIR by providing a structured systems perspective—people, systems, design, and risk—supporting the translation of qualitative insights into actionable pathway design principles, as illustrated in Figure 117. Using CFIR to guide analysis and EBC to inform system-level recommendations enables a nuanced understanding of stakeholder perspectives and contextual constraints, while keeping the focus on practical design choices such as referral criteria, triage processes, access to diagnostics and patient navigation.
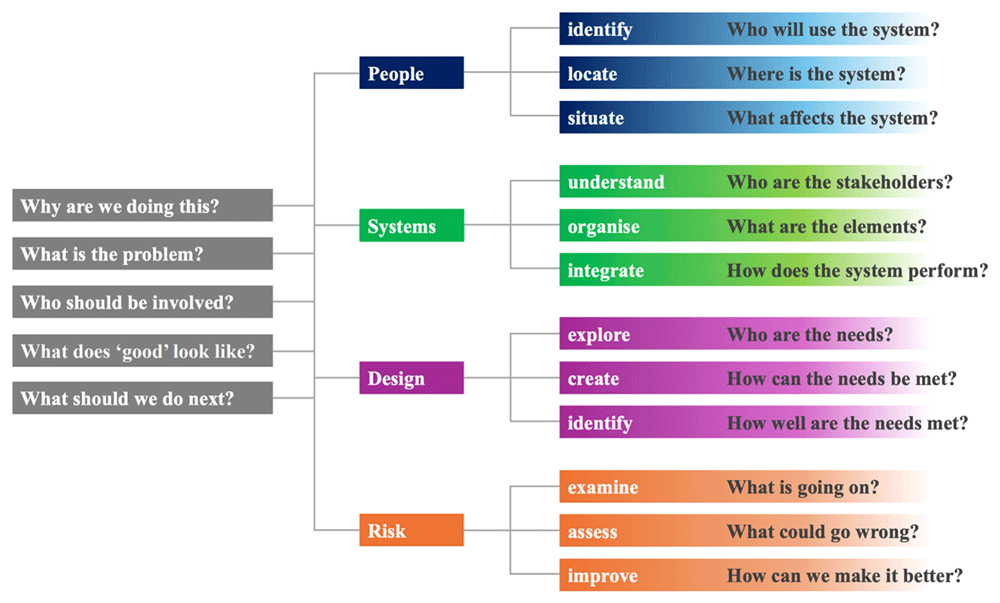
(adapted from reference 17).
This study aims to explore the experiences and perspectives of healthcare professionals, patients and policymakers on the development of a referral pathway for patients presenting with non-specific symptoms of cancer in Irish primary care. The objectives are to identify perceived barriers and facilitators to diagnosing cancer in patients with NSSoC; to understand stakeholder views on the feasibility and acceptability of introducing an NSSoC referral pathway in Ireland; and to explore strategies to support integration of such a pathway into routine primary care practice.
We will conduct a qualitative study using semi-structured interviews to explore determinants, feasibility and design considerations for an NSSoC referral pathway in Irish primary care. The study will be reported in accordance with the Consolidated Criteria for Reporting Qualitative Research (COREQ, 32 items)18. The CFIR will guide data analysis, and the EBC framework will inform topic guide development and the translation of findings into system-level design recommendations16.
CFIR provides a structured taxonomy of intervention characteristics, inner and outer settings, individual characteristics, and implementation processes. We will use CFIR deductively to scaffold coding and matrix development while allowing inductive subthemes to emerge within domains. The EBC framework (people, systems, design, risk) will be applied at the interpretation stage to convert implementation determinants into practical pathway features (especially referral criteria, triage procedures, diagnostic access, communication pathways, and equity safeguards)17. Framework Analysis will be the primary analytic approach, as it is well-suited to applied, policy-relevant questions and supports comparative analysis across stakeholder groups16.
Data collection will take place online to facilitate participation across Ireland, primarily via Microsoft Teams using institutional accounts. Telephone interviews will be offered for participants with limited digital access. The study will be coordinated by the Department of General Practice, RCSI University of Medicine and Health Sciences.
We will include adults (≥18 years) from three stakeholder groups.
Patients will be eligible if they presented with NSSoC within the previous five years and underwent investigation with or without a cancer diagnosis. Individuals who lack the capacity to consent, who would require professional interpretation beyond the study team's capacity, or who are in acute distress at the time of approach will be excluded.
Clinicians will include general practitioners and relevant hospital-based professionals involved in diagnosing cancer in patients with non-specific presentations (e.g., general medicine, oncology, radiology). Clinicians must be practising in Ireland during the study period.
Managers, policymakers and advocacy stakeholders will include service managers, representatives of the National Cancer Control Programme (NCCP) and the Irish College of General Practitioners (ICGP), and representatives of patient advocacy organisations with roles relevant to cancer referral policy or service design.
We aim to recruit approximately 25–30 participants, distributed as follows: 8–10 GPs, 6–8 hospital clinicians, 5–6 managers, policymakers, or advocacy stakeholders, and 6–8 patients. This sampling strategy is flexible and designed to ensure a comprehensive representation of diverse experiences and perspectives, rather than adhering strictly to a predefined numerical threshold for data saturation19–21.
Potential participants will be identified through professional networks (ICGP, NCCP), the Irish Cancer Society and affiliated advocacy groups, academic and service contacts, and announcements at relevant meetings. Interested individuals will receive an invitation pack comprising a study invitation letter, Participant Information Leaflet (PIL), and consent form. Non-responders will receive one reminder approximately two weeks later. Reasons for non-participation, where offered, will be recorded to appraise recruitment barriers. Written informed consent will be obtained electronically before data collection. Where patient participants request support, a caregiver may be present; this will be documented, and the caregiver will not contribute unless explicitly invited by the participant.
Contingency Plan:
We have devised a contingency plan in case we face difficulties with recruitment.
1. In addition to purposive sampling, we will use snowball sampling by asking interviewees to recommend other suitable and willing participants for the study.
2. Expanding recruitment across additional organisations or professional networks if initial numbers prove insufficient.
3. Broadening the scope of eligible participants within each stakeholder group, while maintaining alignment with NSSoC.
4. Extending the recruitment period, if necessary, to ensure adequate response time from participants.
5. Employing multiple dissemination strategies, such as professional newsletters and targeted social media campaigns.
6. Increasing the compensation for participants from 25 Euros.
Suppose recruitment remains below expectations despite these measures. In that case, we will transparently report the limitations in the study findings and adjust the analytical approach to ensure that the diversity and depth of available narratives are accurately represented.
Interviews will be one-to-one, audio-recorded with permission, and are expected to last 30–60 minutes. Topic guides tailored for patients, clinicians, and policymakers will ensure coverage of CFIR domains while incorporating EBC perspectives. Guides will be refined with input from the Patient and Public Involvement (PPI) lead to optimise clarity and sensitivity. We will pilot test the guides with one participant from each stakeholder group and make minor amendments if needed.
Field notes and brief analytic memos will be completed after each interview or group to capture contextual details (e.g., interruptions, affect, rapport) and early interpretive insights. Participants will be offered the option to review their transcripts for accuracy. Any requested corrections will be logged, and the original and revised versions will be retained in the audit trail.
Trained researchers from the RCSI Department of General Practice will conduct interviews. The team includes clinicians and health services researchers with expertise in qualitative methods. Prior relationships with participants are not anticipated; any such relationships will be declared and reflected upon. We will maintain reflexive memos documenting researchers’ assumptions, disciplinary perspectives, and potential influences on data generation and interpretation. The COREQ checklist will report interviewer roles, training and positionality18.
Audio files will be uploaded immediately to encrypted, access-restricted institutional servers and deleted from local devices. Transcription will be undertaken by an approved provider under a confidentiality agreement and verified by the research team. Transcripts will be anonymised by removing names and other identifiers. Only the study team will have access to identifiable data. In line with institutional policy, data will be retained securely for up to 10 years and then destroyed.
Processing of personal data will comply with the General Data Protection Regulation (GDPR) and Irish legislation22,23. The data controller will be RCSI. Participants may withdraw up to two weeks after transcript return; at that point, their data will be removed where feasible.
We will undertake Framework Analysis using NVivo (version 10 or higher). Analysis will proceed through familiarisation, development of an initial analytical framework informed by CFIR domains, indexing (coding) of transcripts, charting into a case-by-theme matrix, and mapping/interpretation. Within each CFIR domain, we will generate inductive subthemes to capture participant-driven insights. At least two researchers will undertake coding. An initial subset of transcripts (approximately 20–30%) will be double-coded to calibrate the framework, followed by periodic cross-checks. Differences will be resolved by discussion, with escalation to a third team member if needed. An audit trail will document framework iterations, coding decisions and reflexive considerations.
Following analysis, we will map priority determinants and candidate strategies to the EBC framework to derive actionable system-level recommendations for pathway design, including equity considerations (e.g., rural access, deprivation), capacity (e.g., imaging slots, laboratory turnaround), and governance (e.g., feedback loops between primary and secondary care).
The PPI lead (CG) is a co-author and contributed to the study conception and the wording of participant-facing materials and topic guides. PPI input will inform recruitment strategies (including accessibility considerations), interpretation of findings (particularly around acceptability and equity), and dissemination products (plain-language summaries and policy briefs). We will report PPI activities and their impact within the manuscript.
This protocol describes a qualitative study to explore the perspectives of general practitioners, hospital specialists, service managers, policymakers, advocacy representatives, and patients on developing a referral pathway for NSSoC in Irish primary care. The study will generate a detailed understanding of barriers and enablers to timely diagnosis, contextual constraints within the Irish health system, and design considerations for a pathway that is both feasible, acceptable, and implementable. A distinctive contribution is the integration of the CFIR framework and the interpretation of findings through the EBC framework, allowing stakeholder insights to shape system-level, actionable design principles.
International evidence demonstrates that patients presenting with NSSoC frequently experience diagnostic delay, repeated consultations, and late-stage cancer diagnosis24. Dedicated diagnostic pathways, such as Denmark’s Diagnostic Centres and England’s non-specific symptoms referral pathways, have reported shorter time to diagnosis, improved patient satisfaction, and high clinician acceptability10,25. However, these evaluations have been largely observational, and relatively little is known about how differences in health system structure, financing, and workforce capacity influence performance.
Ireland presents a distinctive context. The absence of a nationally coordinated NSSoC pathway, coupled with variable access to diagnostic imaging and endoscopy, creates structural inequities in the timeliness of diagnosis14. General practitioners act as gatekeepers to specialist care but report uncertainty about escalation thresholds and limited feedback on referrals. This study will address a vital evidence gap by documenting Irish-specific barriers and facilitators and examining how lessons from international models can be adapted to a mixed public–private health system with marked geographical variation in access.
This will be the first Irish multi-stakeholder study to examine NSSoC referral challenges spanning patients, carers, primary care and secondary care professionals, and system leaders. CFIR enables systematic identification of multilevel determinants, while mapping to the EBC framework supports translation into practical design features (e.g., referral criteria, triage processes, minimum diagnostic bundles, communication loops, navigation support and equity safeguards). The emphasis on systems thinking is particularly relevant given that introducing a new referral pathway will inevitably interact with existing diagnostic capacity, workforce distribution, and policy priorities.
By capturing both professional and patient experiences, the study will also shed light on issues of equity, including whether rural populations, socioeconomically disadvantaged groups, or those with limited health literacy face distinct barriers to timely diagnosis. These insights are essential if the pathway is to avoid inadvertently exacerbating disparities in cancer outcomes.
As with all qualitative studies, findings will be shaped by the perspectives of participants who agree to take part, and self-selection bias may lead to over-representation of stakeholders with a particular interest in service improvement. Despite purposive sampling, some groups—such as patients who disengage from the healthcare system, or clinicians working in the most resource-constrained settings—may be under-represented. The use of online interviews may limit participation among individuals with low digital literacy or poor internet access.
Including patients up to 5 years post-presentation introduces potential recall bias. Excluding participants who require professional interpretation may limit transferability to non-English-speaking populations. Participant compensation may introduce minimal participation bias, although its value and timing are unlikely to affect interview content. Researcher positionality (a GP-led team) will be addressed through reflexive practice, but may shape data generation and interpretation. Finally, the study explores anticipated feasibility and acceptability rather than observing pathway use; policy and capacity contexts may evolve during the study period.
Findings will inform the co-design of a national NSSoC referral pathway by articulating (i) candidate referral criteria and decision-support content for e-referral, (ii) triage models (e.g., centralised versus regional), (iii) a minimum diagnostic bundle (key bloods and cross-sectional imaging access), (iv) bidirectional communication standards between primary and secondary care, and (v) equity safeguards (navigation support; monitoring by HSE region and deprivation quintile). At the policy level, the study will identify enabling conditions for implementation, including protected diagnostic capacity, workforce training, governance arrangements (e.g., NCCP oversight), data flows and a core indicator set (e.g., time to first test, conversion and detection rates, stage at diagnosis, patient-reported experience).
This qualitative phase will inform a prototype pathway and logic model for piloting in real-world settings. Subsequent studies will employ mixed-methods and implementation science designs to assess effectiveness and economic evaluation. Evaluation will include fidelity, feasibility and acceptability measures, alongside hard outcomes (diagnostic interval, stage at diagnosis) and process metrics (triage timelines, imaging turnaround). Comparative work with international models will help understand context–mechanism–outcome relationships and refine transferability.
This study addresses a critical gap in Irish cancer services by exploring how an NSSoC referral pathway might be designed and implemented within primary care. By integrating diverse stakeholder perspectives and applying theory-informed frameworks, the research aims to generate actionable, system-aware recommendations that support earlier diagnosis, more efficient resource use, and equitable access to timely cancer care.
Ethics Approval is being sought from the Irish College of General Practitioners Ethics Review Committee (ICGP_REC_2025_ 3182) Written informed consent will be obtained electronically before data collection. Where patient participants request support, a caregiver may be present; this will be documented, and the caregiver will not contribute unless explicitly invited by the participant.
This protocol will be published on HRB Open, and appendices will be made available via the Open Science Framework.
No data associated with this article.
OSF: Designing a referral pathway for patients with non-specific cancer symptoms in Irish primary care: a qualitative study protocol https://doi.org/10.17605/OSF.IO/TPZJX26 (Invitation letter, participant information leaflet, participant consent form, interview guides and COREQ guidelines checklist are available via the Open Science Framework).
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
COREQ is a 32-item checklist used to assess the quality of qualitative research18.
The authors gratefully acknowledge Sophie Bahadursingh for her assistance with the early draft, Dr Nicholas Clarke for co-helping with the funding application, Dr Una Kennedy for co-supporting the funding application, and the pledge to help disseminate study information to aid in interview recruitment.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with HRB Open Research
Already registered? Sign in
Submission to HRB Open Research is open to all HRB grantholders or people working on a HRB-funded/co-funded grant on or since 1 January 2017. Sign up for information about developments, publishing and publications from HRB Open Research.
We'll keep you updated on any major new updates to HRB Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)