Keywords
Sleep. Dementia. Mild cognitive impairment. Core Outcome Set. Protocol.
While sleep disturbance is common in dementia, leading to negative outcomes, there is growing evidence that sleep disturbance begins early in prodromal dementia and may contribute to cognitive decline. Sleep is therefore an important treatment target throughout the natural history of dementia and there is increasing interest in sleep as a modifiable risk factor. Clinical trials of interventions to improve sleep in people with cognitive impairment are beset by wide heterogeneity in the outcome measures reported. A core outcome set (COS) is urgently required to improve the coherence and comparability of data.
To produce a COS for clinical trials of interventions to improve sleep in people with cognitive impairment: the Sleep in Cognitive Impairment Core Outcome Set (SCICOS).
The Core Outcome Set-STAndards for Development: the COS-STAD recommendations will be followed. A systematic review (Registration: CRD42024556750) will identify outcome measures used in relevant clinical trials. Qualitative interviews involving people living with cognitive impairment and their caregivers will ascertain the outcome measures important to them. Finally, a modified Delphi process, involving people living with cognitive impairment and their caregivers as well as expert clinicians and researchers, will be conducted to reach consensus regarding the final composition of the SCICOS.
Interventions to improve sleep in people with cognitive impairment may reduce distress associated with sleep disturbance and, potentially, slow progression of cognitive decline. Creating the SCICOS will facilitate development of more meaningful and coherent data to drive progress in this emerging field.
Sleep. Dementia. Mild cognitive impairment. Core Outcome Set. Protocol.
In the Methods section, minor changes are made to the paragraph that is sub-headed “Step 1: A systematic review” in order to emphasise more clearly that a detailed protocol for the systematic review has been published elsewhere. Further details regarding the methodology to be employed in the systematic review are available in said protocol.
In the Methods section, the paragraph that is sub-headed “Step 2: Qualitative semi-structured interviews” has been amended to provide some further clarification regarding the eligibility criteria for participation in said interviews. It is clarified that participants will have both cognitive impairment and co-morbid sleep disturbance. Further detail is provided regarding the manner in which the severity of participants’ cognitive impairment will be determined and described. Finally, a sentence is added to explain that, while there may be some concern regarding the ability of people with cognitive impairment to participate reliably in such interviews, it is their own subjective experience and insights that are important to inform the design of the core outcome set.
See the authors' detailed response to the review by Maria Basta
There are approximately 55 million people living with dementia worldwide and, with a rapidly growing and ageing population, the global prevalence of dementia is expected to increase to 139 million by 20501. Despite the recent emergence of disease-modifying therapies2–4, there is no curative treatment. Clinical management, therefore, remains largely focused on addressing modifiable risk factors to prevent development and progression of prodromal dementia, and on reducing the symptom burden in more advanced cases.
Sleep disturbance affects up to 70% of people with cognitive impairment5 and contributes to worsening cognitive outcomes5, impaired daytime functioning and deteriorating quality of life6. The increased carer burden caused by sleep disturbance in people with dementia has been shown to lead to sub-optimal care7 and early institutionalisation8, which increases the economic burden of dementia care9.
There is also increasing evidence that sleep disturbance may play a role in precipitating the onset and accelerating the progression of cognitive decline10. At a biological level, for example, there is evidence that sleep disturbance may increase accumulation within the cerebrospinal fluid of the beta-amyloid and tau proteins that are biomarkers of Alzheimer’s disease11. Although the recently published Dementia Prevention, Intervention and Care: 2024 report of the Lancet Commission12 has reaffirmed that additional research is required to definitively clarify the effect of sleep on cognitive decline, interest is nevertheless growing in sleep as a modifiable risk factor for cognitive impairment13.
Despite the importance of sleep as a treatment target throughout the natural history of dementia, to potentially slow disease progression in prodromal stages and to reduce the burden of symptoms in more advanced cases, the evidence base supporting clinical practice is limited14. One review notably concluded that “the literature available to guide effective treatment of sleep in patients with Alzheimer’s disease is woefully insufficient”6. Systematic reviews of interventions to improve sleep in people with cognitive impairment have been limited by small sample sizes with wide heterogeneity, both in the manner in which sleep is measured and in the outcome measures reported, limiting data synthesis15,16.
A 2022 scoping review of the measurement of sleep in people with mild cognitive impairment (MCI) and mild dementia found that, after synonymous terms had been combined, 165 separate sleep outcomes were reported across all included studies17. This heterogeneity limits comparability of data, which precludes data synthesis and inhibits the development of a robust evidence base to guide clinical practice. Furthermore, 62 (37.6%) of the 165 sleep-related outcomes were reported in only one included study, raising the possibility of positive-finding publication bias.
In addition to this heterogeneity in the outcome measures reported, the aforementioned scoping review17 demonstrated significant variability in the methods used to measure sleep. Of the 188 studies included in the review, 131 used subjective measures of sleep such as questionnaires or sleep diaries. Objective measures of sleep were used less frequently, with polysomnography being used in 88 and medical-grade actigraphy being used in 37 of the included studies. It is, perhaps, unsurprising that most of the studies used subjective measures of sleep, given their low cost, ease of administration and the relatively low burden imposed on participants17A. However, discrepancies between subjective and objective measures of sleep in people with cognitive impairment are well documented17B–17C. While the subjective experience of sleep is an important outcome measure in its own right, subjective measures of sleep are undoubtedly susceptible to recall bias, which is likely to be particularly problematic among people with cognitive impairment17D. Polysomnography is widely recognised as the gold standard method of measuring sleep17E. However, it is relatively expensive, requires expertise for interpretation, and the more invasive nature of the assessment – generally requiring overnight stays in sleep laboratories whilst wearing multiple attachments to measurement equipment – may be unsuitable for people with more advanced cognitive impairment17D. There are several other methods of measuring sleep objectively, each with its own associated advantages and disadvantages17F and varying degrees of accuracy compared to gold standard polysomnography17G. Acknowledging that there is no direct one-to-one relation between the different methods of measuring sleep, subjective and objective measures likely make their own uniquely valuable contributions to sleep quantification17H. The lack of concordance between them, however, further limits opportunities to compare data from clinical trials that use different methods of measuring sleep.
A core outcome set (COS) is therefore urgently required to improve the coherence and utility of research data in the field. A COS is an agreed standard or minimum set of measurements and outcomes that all studies in a particular field of research are recommended to report18. This study aims to involve key stakeholders, including people living with cognitive impairment and their caregivers, in the creation of a COS for clinical trials of interventions to improve sleep in people with cognitive impairment – the Sleep in Cognitive Impairment Core Outcome Set (SCICOS). The SCICOS will increase clinical trial efficiency, reduce the risk of reporting bias, improve data synthesis and help build a robust evidence base for interventions that improve the lives of people living with cognitive impairment and their caregivers.
The SCICOS has been registered on the Core Outcome Measures in Effectiveness Trials (COMET) database (see: https://www.comet-initiative.org/Studies/Details/3250) and will be created in accordance with the Core Outcome Set - STAndards for Development (COS-STAD) recommendations from the COMET Initiative19. These recommendations will, however, be adapted to facilitate participation of people living with cognitive impairment and their caregivers, as suggested by the CO-research Involvement and Engagement in Dementia (COINED) model20. The SCICOS study design will therefore involve a multi-stage mixed methods approach in four steps, as follows (see Figure 1):
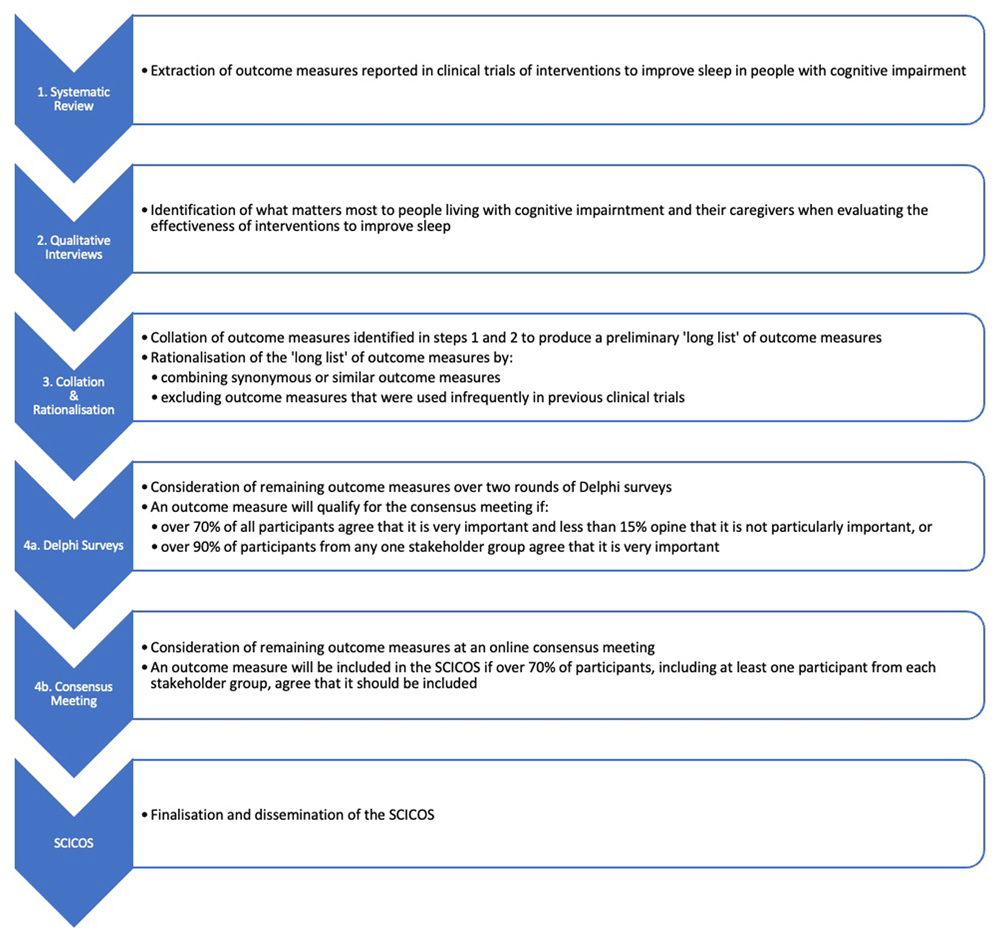
SCICOS = Sleep in Cognitive Impairment Core Outcome Set.
Step 1: A systematic review will be undertaken of randomised and non-randomised controlled clinical trials involving both pharmacological and non-pharmacological interventions to improve sleep in people with cognitive impairment, encompassing those with MCI and dementia. A detailed protocol for the systematic review has been published elsewhere21 and has been registered with the International Prospective Register of Systematic Reviews (PROSPERO), registration number: CRD42024556750. The systematic review will produce a list of all the outcome measures reported in the included trials.
Step 2: Qualitative semi-structured interviews will be conducted among a convenience sample of 20 – 25 people living with cognitive impairment and co-morbid sleep disturbance, and/or their caregivers, to examine what matters most to them when determining the effectiveness of an intervention to improve sleep. The interviews will therefore endeavour to ascertain the outcome measures that people living with cognitive impairment and their caregivers deem most important in any clinical trial of an intervention designed to improve sleep. Purposive sampling will be used to include people living with different stages of cognitive impairment21A, as determined by a consultant physician in geriatric medicine. In this regard, the Reisberg FAST criteria will be used to describe the severity of participants’ cognitive impairment21B. While there may be some concern regarding the ability of people with cognitive impairment to participate reliably in these interviews, it is nevertheless their own subjective experience and insights that are sought. When developing a core outcome set, it is important to garner the opinions of people with lived experience of the medical condition being examined in this manner, as they may indicate additional outcomes of importance or emphasise different priorities compared to those suggested by clinicians or researchers22. Participants will be drawn from patients and, if required, their caregivers attending memory clinics at the Mercy University Hospital in Cork, Ireland. They will also be drawn from the Patient and Public Involvement (PPI) panels of Dementia Trials Ireland (DTI), a Clinical Trials Network funded by the Health Research Board of Ireland. Prior informed written consent will be obtained from all participants. A bespoke interview topic guide will be used (see Table 1 in extended data). Responses will be recorded and transcribed verbatim. Qualitative data analysis software will be used to analyse the transcripts in accordance with the reflexive thematic analysis approach espoused by Braun and Clarke23.
Step 3: Collating the results of the systematic review and the qualitative semi-structured interviews. Any additional outcome measures suggested by the qualitative semi-structured interviews (described in step 2 above) will be collated with the list of outcome measures produced by the systematic review (described in step 1 above) to produce a preliminary ‘long list’ of outcome measures. These initial outcome measures will be categorised by domain and core area in accordance with the OMERACT Filter 2.0 Framework24. Outcome measures within the same domain that are synonymous or similar may be combined to avoid unnecessary duplication. The categorisation and combination of outcome measures in this manner will be undertaken independently by two researchers and any disagreements will be resolved by the principal investigator, as recommended by the COMET Handbook25.
A previous scoping review examining studies involving the measurement of sleep in people with MCI and mild dementia has demonstrated that many outcome measures appear in only one (37.6%) or two (58.8%) studies17. As any outcome measure that appears so infrequently is unlikely to be considered a core outcome measure, we will exclude from further consideration in the modified Delphi process any outcome measure that:
(a) appears in only one clinical trial, or
(b) appears in any number of clinical trials which, when combined, involve less than 1% of the total number of participants involved in all the clinical trials included in the planned systematic review, described above.
Step 4: A modified Delphi process will be employed to reach consensus regarding the outcome measures that should be included in the SCICOS25. There is no consensus on the recommended sample size for a Delphi panel25. We aim to include sufficient lay and professional participants to ensure comprehensive representation of different perspectives at all stages of the process25. Different modes of participation in the modified Delphi process will be offered to professional and lay participants in order to facilitate involvement of people living with cognitive impairment and their caregivers26. Fully informed prior written consent to participation will be obtained from all participants. Demographic details, including age, sex and stakeholder group (either professional or lay participants) will be collected.
Professional participants will have an established research interest in sleep and/or dementia. They will be drawn primarily from among the authors of research papers that are included in the systematic review mentioned in step 1 above. Professional participants will be invited to participate in the modified Delphi process by completing an electronic survey administered via an online platform.
Lay participants will be people living with cognitive impairment or their caregivers. They will be drawn from the PPI panel of DTI and from patients attending memory clinics at the Mercy University Hospital in Cork, Ireland. To facilitate meaningful involvement of people living with cognitive impairment and their caregivers in the modified Delphi process, lay participants will be offered different modes of completing the Delphi surveys26. Lay participants may complete the Delphi surveys via paper-based survey and may avail of the assistance of a researcher to do so via a researcher-supported survey. A researcher-supported survey involves a researcher supporting the participation of the person living with cognitive impairment by helping them to complete the survey26.
The modified Delphi process will consist of two ‘rounds’ of surveys, in which participants are asked to rate the importance of each outcome measure, before a final consensus meeting is convened among a sub-set of participants to determine which outcome measures will be included in the SCICOS. In line with the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE)27, professional participants will be asked to score the importance of each outcome measure on a 9-point Likert scale28. Subsequently, in order to align with the scores of lay participants (see below), scores from 1 to 3 will be defined as ‘not particularly important’, scores from 4 to 6 will be defined as ‘important but not critical’ and scores from 7 to 9 will be defined as ‘very important’.
Each outcome measure included in the lay participants’ Delphi survey will be accompanied by an ‘accessible statement’, which is a simple, clear and concise description of the outcome measure in plain non-technical language29. The Likert scale will also be adapted in the lay participants’ Delphi survey25. Previous research has demonstrated that Likert-scales with five or more points are unsuccessful in people with dementia30. Instead, therefore, a three-point scale will be used: ‘not particularly important’, ‘important but not critical’, and ‘very important’26.
The following criteria will be applied in order to determine which outcome measures qualify for consideration at the final consensus meeting. In each of the two rounds of Delphi surveys, outcome measures will qualify for consideration at the final consensus meeting if at least 70% of all participants regard them as ‘very important’ and less than 15% regard them as ‘not particularly important’, or if over 90% of participants from one stakeholder group regard them as ‘very important’31. After the first Delphi survey round, outcome measures will be excluded from further consideration in the second round if at least 70% of all participants regard them as ‘not particularly important’ and less than 15% regard them as ‘very important’. During the second Delphi survey round, the results of the first round will be available to participants for consideration and they will be asked if they would like to change how they rated each remaining outcome measure in light of this information.
Following the second Delphi survey round, the final consensus meeting will be convened virtually to determine the ultimate composition of the SCICOS. All participants in the two Delphi survey rounds will be invited to participate in the final consensus meeting. Outcome measures will be considered at the final consensus meeting if, in either of the two preceding Delphi survey rounds, at least 70% of all participants rated them as ‘very important’ and less than 15% rated them as ‘not particularly important’, or if over 90% of participants from one stakeholder group regarded them as ‘very important’31. At the final consensus meeting, each such outcome measure will be considered in turn and will be included in the SCICOS if at least 70% of participants, including at least one person from each stakeholder group, votes in favour of its inclusion in the SCICOS. Additionally, participants will be invited to give their opinion regarding the most appropriate method of measuring each outcome measure that is included in the SCICOS. In this way, the SCICOS will not only prescribe a core set of outcome measures for clinical trials of interventions to improve sleep in people with cognitive impairment, but will also make recommendations regarding the most appropriate method of measuring those outcome measures. This will help to reduce problems associated with the lack of concordance between the different methods of measuring sleep.
The study has commenced and is currently on-going.
In recent years, due to the growing burden of dementia1 and an increasing preponderance of evidence implicating sleep disturbance not just in its symptomatology but also perhaps in its pathophysiology10,11, there is growing research interest in the treatment of sleep disturbance in people with cognitive impairment13,17.
Most clinical trials of interventions to improve sleep in people with cognitive impairment have been limited by small sample sizes15,16. The synthesis of data from these trials has been limited in turn by wide heterogeneity both in the methods used to measure sleep and in the outcome measures reported, precluding meta-analysis16 and impeding the development of a reliable evidence base to guide clinical practice. Given the challenges inherent in conducting clinical research among people with cognitive impairment32, it is important to ensure that future research is reliable and coherent, thus increasing the likelihood that the research will lead to clinically meaningful insights. The SCICOS will improve the utility and efficiency of future clinical trials by prescribing a standard minimum set of outcome measures, and making recommendations regarding the methods used to measure them, thus reducing risk of reporting bias and facilitating meta-analysis.
The SCICOS will be developed in accordance with the COS-STAD recommendations19. Clinicians and researchers from around the world with established expertise in sleep and/or dementia will be invited to participate to optimise generalisability, credibility and relevance. The COS-STAD recommendations will be adapted to ensure meaningful involvement of people living with cognitive impairment and their caregivers throughout the process of developing the SCICOS. A 2020 Cochrane review of pharmacotherapies in dementia33 found that clinical trials do not always succeed in taking account of what matters most to the end-users of the tested interventions. In ancillary qualitative research among caregivers of people with dementia, it was noted that their highest priority outcome was uninterrupted sleep at night yet this was not included as an outcome measure in any of the included trials. The process for developing the SCICOS includes people living with cognitive impairment and their caregivers as key stakeholders.
As a practical consequence of the adaptations made to the study design to facilitate meaningful involvement of people living with cognitive impairment and their caregivers, data collection from this key stakeholder group will be limited to Ireland. This may potentially introduce bias, as the level of importance attributed to outcome measures may vary across different countries and cultures. Nevertheless, by involving people living with cognitive impairment and their caregivers throughout the process of creating the SCICOS, this study will ensure that the voices of this key stakeholder group will be heard in all relevant future clinical trials.
There is growing interest in sleep disturbance as a modifiable risk factor for cognitive impairment. Sleep disturbance remains an important treatment target in more advanced dementia to alleviate the associated symptom burden and carer strain. Existing literature regarding the role of sleep in cognitive impairment is beset by wide heterogeneity in the methods used to measure sleep and in the outcome measures reported. Creating a COS for clinical trials involving interventions to improve sleep in people with cognitive impairment will increase comparability of data and help build the evidence base for interventions that can improve the lives of people living with cognitive impairment and their caregivers.
The SCICOS study will be conducted in accordance with the Declaration of Helsinki and General Data Protection Regulations (GDPR). Ethical approval was granted for each phase of this study by the Clinical Research Ethics Committee of the Cork Teaching Hospitals (CREC) on 30th July 2024, reference numbers: ECM 4 (c) 06/02/2024 & ECM 5 (7) 20/02/2024 & ECM 4 (dd) 30/07/2024.
Fully informed written consent will be obtained from all participants prior to their participation in the study. In order to facilitate the process of obtaining fully informed consent, each prospective participant will be provided with a comprehensive Participant Information Leaflet that is appropriate to their personal circumstances. Separate versions of the Participant Information Leaflet will be provided to prospective participants from each stakeholder group, namely professional and lay participants. Within the latter stakeholder group, separate versions of the Participant Information Leaflet will be provided to people living with cognitive impairment and their caregivers. Copies of each version of the Participant Information Leaflet and Consent Form are provided in the extended data.
The SCICOS will be reported in accordance with the Core Outcome Set-STAndards for Reporting: the COS-STAR Statement34. Everybody who participated in the process of developing the SCICOS will be provided with a copy of the final report. The SCICOS will be disseminated through submission for publication in a peer reviewed medical journal and promulgation at relevant clinical conferences.
Open Science Framework: Protocol for the creation of a core outcome set for clinical trials of interventions to improve sleep in people with cognitive impairment: The Sleep in Cognitive Impairment Core Outcome Set (SCICOS) study. https://doi.org/10.17605/OSF.IO/U96RJ
This article contains the following extended data35:
Open Science Framework: Protocol for the creation of a core outcome set for clinical trials of interventions to improve sleep in people with cognitive impairment: The Sleep in Cognitive Impairment Core Outcome Set (SCICOS) study. https://doi.org/10.17605/OSF.IO/U96RJ
This article contains the following reporting guidelines35:
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 universal).
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Partly
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: sleep medicine , geriatric psychiatry
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Partly
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: I am a sleep researcher with a focus on the role of sleep and biological rhythms in brain health
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Partly
Are sufficient details of the methods provided to allow replication by others?
Partly
Are the datasets clearly presented in a useable and accessible format?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Alzheimer's disease and pharmaceutical analysis
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 3 (revision) 09 Jan 26 |
|||
|
Version 2 (revision) 06 May 25 |
read | ||
|
Version 1 28 Oct 24 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with HRB Open Research
Already registered? Sign in
Submission to HRB Open Research is open to all HRB grantholders or people working on a HRB-funded/co-funded grant on or since 1 January 2017. Sign up for information about developments, publishing and publications from HRB Open Research.
We'll keep you updated on any major new updates to HRB Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)