Keywords
Hidradenitis suppurativa, dermatology, CD14, biomarker, LPS, inflammation
Hidradenitis suppurativa, dermatology, CD14, biomarker, LPS, inflammation
Hidradenitis suppurativa (HS) is a highly prevalent inflammatory skin disease, affecting 1–4% of the general population1. It has a significant impact on patient quality of life, worse than other common conditions such as psoriasis and atopic dermatitis, with patients often suffering from multiple psychological co-morbidities such as depression2. HS is characterized by recurrent abscesses which derive from the rupture of occluded hair follicles, but the aetiology of the disease is still poorly understood. It is known that the immune system is dysregulated in multiple ways in the skin and blood of HS patients1. Pro-inflammatory cytokines such as interleukin (IL)-1β and IL-6 are elevated in HS patients and may result from increased infiltration of monocytes and macrophages into the skin3,4. The causes of this immune dysregulation are unknown, but there is increased recognition that systemic factors may play a role in the disease. Crohn’s disease is a major co-morbidity of HS and is associated with significant impairment of the gut epithelial barrier5 termed "leaky gut". The gut and skin microbiomes of HS patients have been shown to be dysregulated, pointing towards a disrupted immunity barrier and a deleterious relationship between host and microbe in these patients6–9. A leaky gut allows bacterial translocation from the gut lumen into circulation, leading to increased circulation of bacterial lipopolysaccharide (LPS)10. LPS is an agonist of innate immune receptors such as Toll-like receptor (TLR) 4, activating signalling pathways which result in pro-inflammatory cytokine release. LPS is presented to TLR4 via the adaptor molecules LPS-binding protein (LBP) and CD1411. In response to the presence of excess LPS, monocytes can release CD14 in a soluble form (sCD14) which is a well described biomarker in diseases with barrier impairments10. Diseases that feature elevated patient sCD14 levels include acute and chronic conditions such as sepsis and HIV respectively, and notably the inflammatory skin condition atopic dermatitis12–17. CD14 release from cell membranes in sepsis is governed by P2X7 receptor, which is also elevated in HS18. CD14 is primarily expressed by myeloid cells, such as monocytes and macrophages, which are overrepresented in HS patients3. Given the dysregulation of the microbiome, myeloid cell compartment and cutaneous inflammation associations we hypothesized that patients with HS have a leaky gut barrier. To address this, we sought to determine the levels of sCD14 present in the serum of HS patients as a proxy marker.
The study was approved in advance by the Ethics and Medical Research Committee of St Vincent’s University Hospital (approval date 2/10/2019 and 11/02/2020). Written, signed informed consent was obtained via signature from each participant for sample collection, data collection and publication of results. Patient data was processed at sites covered under ethics approval provided by the Ethics and Medical Research Committee of St Vincent’s University Hospital. For this study, the data was de-identified using the Safe Harbour method and is available via Open Science Framework19.
Blood was collected from 17 HS patients as part of their routine clinical visits to St Vincent’s University Hospital, and from nine healthy volunteers at the Charles Institute of Dermatology, University College Dublin. Samples were collected during monthly clinics between September 2020 and March 2021 at once off appointments with no follow-up. Healthy controls were deemed eligible if they were healthy and did not suffer from any inflammatory or infectious disease. HS patients were deemed eligible upon attendance of the HS clinic at Charles Centre for Dermatology, St Vincent’s University Hospital to treat their condition. HS patients recruited were not excluded based on current or previous therapeutic regimens. Of the HS patients, 41% were not undergoing treatment for their HS, 24% were being treated with anti-TNF biologics (adalimumab and infliximab), 6% were being treated with anti-IL-17 antibodies (brodalumab), 18% were being treated with Rifampicin and clindamycin and 12% were treated with other antibiotics (lymecyclin and sprinolcatone). 30% of HS patients were smokers, 35% were ex-smokers and 35% were non-smokers. Of the healthy controls, 77% were non-smokers, 11% were current and 11% were ex-smokers. The study size was determined with reference to similar studies in the literature with similar estimated effect sizes4,20. A total of 5 ml of patient blood was collected using the BD Vacutainer system into BD Serum collection tubes (Becton, Dickinson U.K. Limited). These tubes were allowed to rest for 30 min at room temperature before centrifugation at 10,000 rpm for 10 min at 4°C. The serum was removed from the clot and immediately aliquoted in fresh polypropylene tubes and frozen at -80°C. Immediate freezing and aliquoting was done to remove day-to-day variability between sample collections and to prevent unwanted freeze-thaw artefacts, respectively. Once the sCD14 was measured in healthy control and HS serum by enzyme linked immunosorbent assay (ELISA) using Quantikine sCD14 ELISA kits (RnDSystems, Abingdon, UK). Serum was diluted 1 in 200 in reagent diluent included as part of the kit as per the manufacturer’s instructions. Absorbance at 450 nm was detected using a SpectraMax M3 plate reader with SoftMax Pro Version 6 analysis software (Molecular Devices, San Jose, CA, USA). Statistical analysis of the difference between the mean sCD14 levels of the control group and the HS group was performed using GraphPad Prism Version 9.00 (GraphPad Software, La Jolla, CA, USA.) and an unpaired Student’s t-test with significance of P <0.05 and correlation analysis using data recorded as part of the patient’s routine clinical examination or self-reported by healthy controls was performed using Spearman’s rank correlation co-efficient. Underlying data are available as a .csv file for analysis using SPSS and R can be used as an alternative open-source statistical analysis software19. Where patient data was incomplete (e.g. some HS BMI measurements) it has been indicated in the figure legends for clarity.
The mean serum level of sCD14 was not significantly different in patients with HS (5.021 ng/ml ± 2.223, n = 17) when compared to healthy controls (3.680 μg/ml ± 1.691, n = 12, P = 0.09). This result is presented in Figure 1, and the accompanying patient demographics are presented in Table 1. In addition, to rule out differences in body mass index and age (BMI) which may impact interpretation of the results, sCD14 levels were correlated against BMI and age where this data was available. No significant correlations were observed, as presented in Figure 2. In addition, no correlation was found between sCD14 levels and C-reactive protein (CRP) levels measured at time of serum sampling as presented in Figure 3.
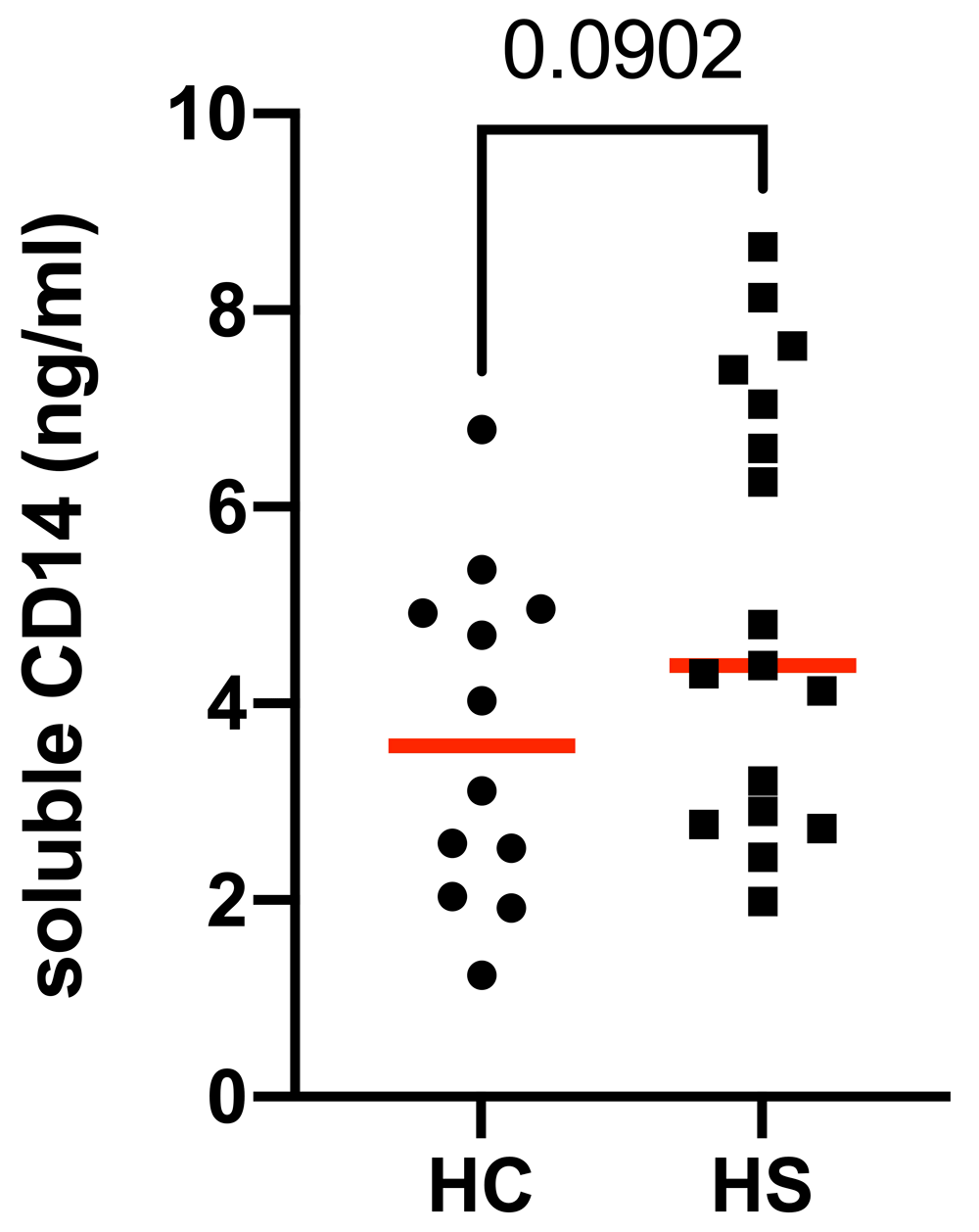
Dot plot represents individual samples (HS=square, HC=circles) with mean levels of soluble CD14 levels (red line) in each group. P=0.0902 by unpaired Student’s t-test.
| N | Age [years] | BMI | Female | Male | |||
|---|---|---|---|---|---|---|---|
| mean | range | mean | range | ||||
| HS | 17 | 38 | 27–54 | 35 (n=10) | 24–54 (n=10) | 13 | 4 |
| HC | 12 | 31 | 21–55 | <25 | 22–26 | 7 | 5 |
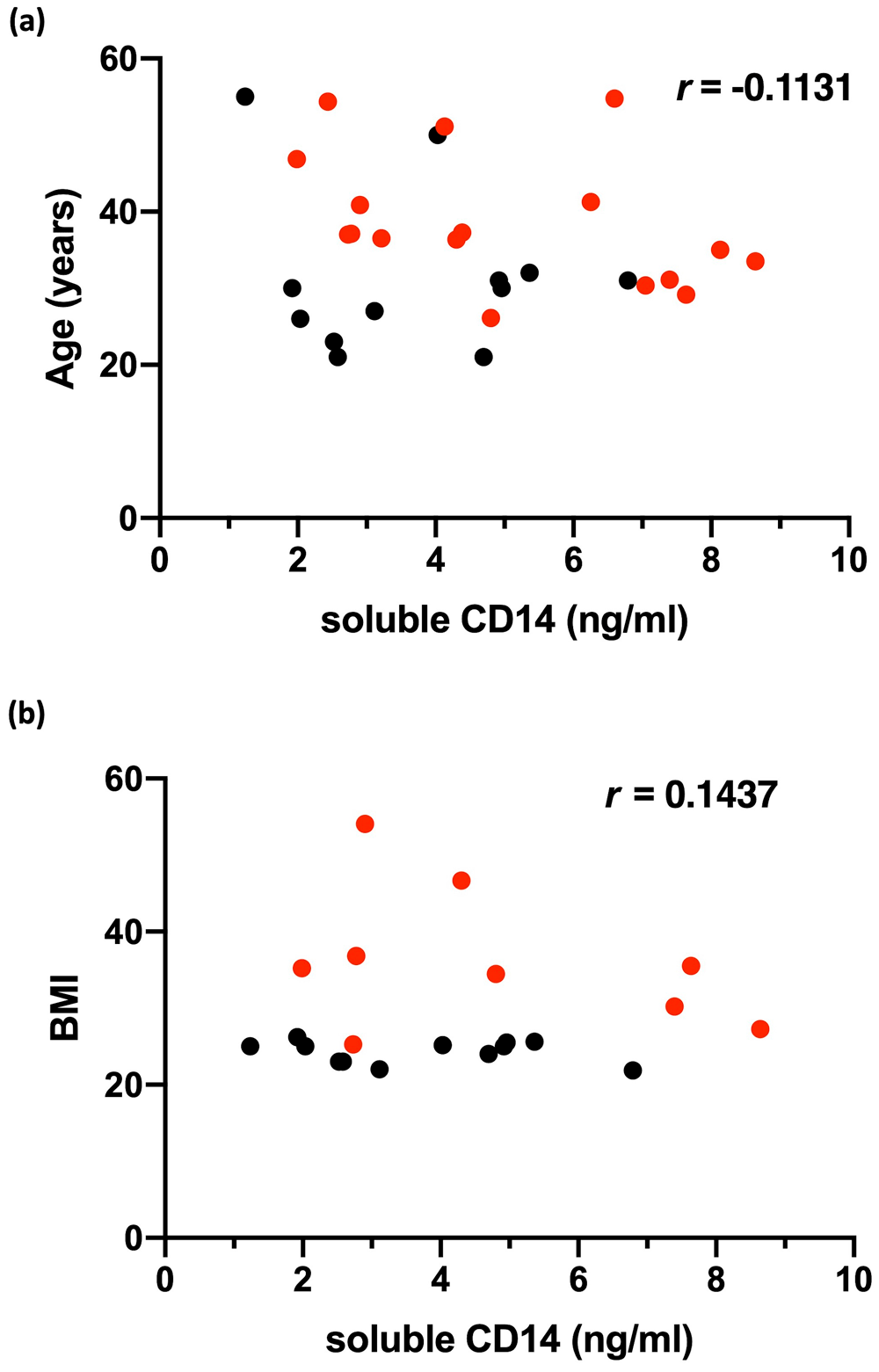
Correlation analysis of soluble CD14 levels with (a) age and (b) BMI in healthy controls (HC) and patients with hidradenitis suppurativa (HS). For b), n=9 and 10 respectively. Dot plot represents individual samples (HS=red, HC=black). r values derived from Spearman’s rank correlation co-efficient test.
Our data demonstrate no statistically significant increase in sCD14 in HS patients, nor any correlation with age, BMI or circulating CRP levels. The clearest conclusion to draw from this study is that elevated sCD14 does not serve as an indicator of leaky gut in HS patients. This may be due to the absence of leaky gut in this condition, the presence of alternative redundant mechanisms, or a lesser degree of “leakiness” relative to other conditions. It is interesting in the context of the reported microbial dysregulation in the gut and skin of HS patients and may be a reflection of a low level of gut barrier disruption over a chronic time period as distinct from a more acute event. Commensal microbial involvement in inflammatory disease is complex and multi-faceted, and these results may point towards altered host:microbe interactions in non-innate immune compartments such as T and B cell responses. Several studies have demonstrated an expansion of plasma and B cell numbers in HS, as well as reports of elevated anti-fungal antibody titres that might point towards one such host:microbe interaction21–25. Given the p value reported when comparing HS sCD14 levels to that of healthy controls, it could be concluded that this study is not large enough and includes too few patients. However, we believe that the study is sufficiently powered to capture any significant differences according to previously reported effect sizes16,17. This may not be the case for correlation analysis, which may indeed require a larger study to properly interrogate. One limitation of this study is the fact that most of the patients (15/17) were Hurley Stage 2, which does not allow for analysis by disease stage. It would be interesting to test this hypothesis in a larger cohort of patients of varying disease severity, and with additional co-morbidities such as Crohn’s disease. In summary, these data do not indicate that sCD14 levels are indicative of a leaky gut in HS patients, but do not rule out a link between the disease and gastrointestinal dysfunction.
Written informed consent for publication of the patients’ details was obtained from the patients.
Open Science Framework: Underlying data for ‘A preliminary study of soluble CD14 levels in the serum of patients with hidradenitis suppurativa as a marker of “leaky gut”’. “sCD14 in hidradenitis suppurativa”. https://doi.org/10.17605/OSF.IO/C9Y2W19.
This project contains the following underlying data:
Data file 1: HRB-OR_sCD14_ELISA.csv - raw data in open-source CSV format
Data file 2: HRB-OR_sCD14_ELISA.xlsx - raw data in Microsoft Excel format
Data file 3: HRB-OR_sCD14.pzf - processed data in GraphPad Prism format
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0)
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Inflammatory cytokines, Crohn's disease, Ulcerative colitis, Hidradenitis suppurativa
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Autoinflammatory skin diseases; genodermatoses; genetics.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 25 Oct 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with HRB Open Research
Already registered? Sign in
Submission to HRB Open Research is open to all HRB grantholders or people working on a HRB-funded/co-funded grant on or since 1 January 2017. Sign up for information about developments, publishing and publications from HRB Open Research.
We'll keep you updated on any major new updates to HRB Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)