Keywords
Cardiovascular Diseases; Primary Prevention; High Risk Patients; Feasibility Studies; General Practice
Cardiovascular Diseases; Primary Prevention; High Risk Patients; Feasibility Studies; General Practice
Cardiovascular disease (CVD) is the main cause of deaths worldwide with approximately 18 million people dying from the condition annually1. According to the Centers for Disease Control and Prevention (CDC), notable CVD risk factors include pre-existing health concerns such as high blood pressure, unhealthy cholesterol levels, diabetes, behavioural / lifestyle factors such as poor diet, physical inactivity, alcohol / tobacco use, and social determinants, particularly low socioeconomic status.
The advent of programmes to systematically promote the identification, assessment, and treatment of patients at risk of CVD are especially welcome. One example of such a programme is Ireland’s ‘Chronic Disease Management Programme’, which is in its first phase, and is targeting patients who are eligible for free General practitioner (GP) care and who are aged over 75 years with Diabetes Type 2, Asthma, Chronic Obstructive Pulmonary Disease (COPD) and CVD (including heart failure, ischaemic heart disease, cerebrovascular disease, and atrial fibrillation). As part of the programme, patients have two scheduled reviews with the Practice Nurse (PN) in a 12-month period to include patient education, preventative care, medication review, physical examination, investigations, and an individual care plan.
Whilst initiatives such as this are an appreciated addition, a more intensive approach is required to meet the ongoing care needs of the most high-risk patients. To that end, the High-Risk Prevention Programme (HRPP), led by the Irish Heart Foundation in collaboration with Ireland’s Health Service Executive (HSE) aims to design, deliver, and evaluate the feasibility of an intensive, six-week behaviour change programme within general practices for people at high risk of CVD, many of whom are living in deprived communities. Research shows that targeted interventions of this nature show much promise, and that factors such as appropriate funding / resources, high accessibility, opportunistic care provision, and adequate staff training are likely to yield positive outcomes for patients, communities, healthcare staff, services, and policymakers2,3.
Nevertheless, there is consensus that cross-cultural differences should be accounted for when designing and implementing interventions, that a large variety of intervention approaches have subsequently been used for culturally specific high-risk populations to date, and that this variety has become something of a barrier to determining a universal comprehension of intervention effectiveness4. With these considerations in mind, continued evaluation of more specialist interventions, those which are conducted with the unique care needs and sociocultural contexts of targeted population sub-groups in mind, is required. The HRPP initiative therefore aims to advance knowledge regarding the unique care needs and requisite care response for high-risk CVD patients living in Irish communities, many of them deprived, and the project’s findings and recommendations will have the potential to serve as key evidence supports for CVD-related health and social care clinical and research practices in Ireland going forward.
Specifically, the study proposed in this protocol aims to evaluate the HRPP project by way of:
Determining its likely effectiveness in terms of enhancing patients’ health, health behaviour, health knowledge, and motivation to improve health.
Assessing its acceptability to patient and service provider stakeholders by investigating their views and experiences of the initiative.
Examining quantitative metrics outlining patient engagement with the programme.
Exploring environmental factors that may facilitate and / or hinder health within participating practices’ communities.
The HRPP’s design process involving a co-design initiative with participating general practices, patients, the Irish Heart Foundation, and HSE representatives began in late 2020. Programme delivery and evaluation activities have been operating in collaboration with researchers from University College Dublin (UCD) since February 2021, and the programme will continue for 48 months, ending in February 2023. This study and protocol are informed by the ‘CONSORT 2010 Statement: extension to randomized pilot and feasibility trials’5, the ‘Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)’ guidelines6, and Lancaster and Thabane’s (2019) recommendations regarding the dual application of these frameworks7. The completed CONSORT and STROBE guidelines, and the informed consent form and study instruments can be found as Extended data8.
General practices were recruited by means of an invitation for expressions of interest sought through the Foundation website, the Medical Independent (a medical news website for doctors, healthcare professionals, and people with an interest in health issues), and the Foundation’s social media channels (Twitter, LinkedIn, and Facebook). Six practices, four in Dublin and two in Wexford, were selected to be representative in terms of practice size, urban, rural, and mixed urban / rural spread, and willingness to participate in the previously mentioned co-design process.
Intervention / study participants included healthcare personnel working at the selected practices and adult patients who:
Were aged 40 years or older,
Were in possession of a General Medical Services (GMS) or doctor visit card,
Had been identified as being at high risk of CVD, including hypertension, high cholesterol, physically inactive, overweight / obese, and Type 2 diabetes patients with no access to an alternative lifestyle behaviour change programme, as well as patients with 10-year Q-Risk scores >20%. Patients who smoke were signposted to appropriate smoking cessation services for support. If included in the HRPP with other risks their progress was monitored.
Had been deemed physically and mentally well enough by healthcare professionals to provide informed consent and to participate in the programme.
Patients were not eligible for the programme if:
They had an already established CVD, e.g., coronary artery disease, history of stroke / transient ischaemic attack (TIA), heart failure,
They had Type 2 diabetes and were already availing of a lifestyle behaviour change programme,
<40 years,
They were deemed unable to self-manage their health, e.g., dementia patients,
They did not have a GMS or doctor visit card.
Patients attending participating practices were invited to take part in the study on an ongoing basis by each practice via phone call. The project targets recruiting approximately 6–12 patients at each practice per six-week cycle, and there will be eight cycles in total. Thus, by completion of the project, it is hoped that approximately 400 patients will participate, a figure that has been deemed sufficient for the purposes of obtaining required statistical power for evaluation-based quantitative analysis.
Intervention overview. The HRPP intervention involves a two-arm pre-post design whereby recruited patients attend either a PN or Health Promotion Coordinator (HPC) led six-week one-to-one consultation programme focusing on self-management of health issues. The intervention also consists of two PN / HPC administered patient assessments involving the conduct of routine physical health tests and survey interviews examining patients’ self-reported health behaviours, mental health, health knowledge, and motivation to be healthier (prior to commencing the six-week programme (Time 1) and 12 months following its completion (Time 2)). These assessments serve a dual purpose as they facilitate clinical level assessment and subsequent strategies for individual patient care, as well as data collection for evaluation purposes. Participant interactions with PNs and the HPC occurred in-person and virtually in line with COVID-19 restrictions.
Participating patients also receive confidential follow-up calls involving discussions around health behaviour maintenance, reviewing progress, and supporting sustainable change from PNs/HPC at three, six, and nine-months following completion of the programme. Some patients are invited to take part in semi-structured interviews shortly after completion of the six-week programme, whereby they can describe in detail their experiences of the programme and whether, and how, they feel it can be improved (see Figure 1).
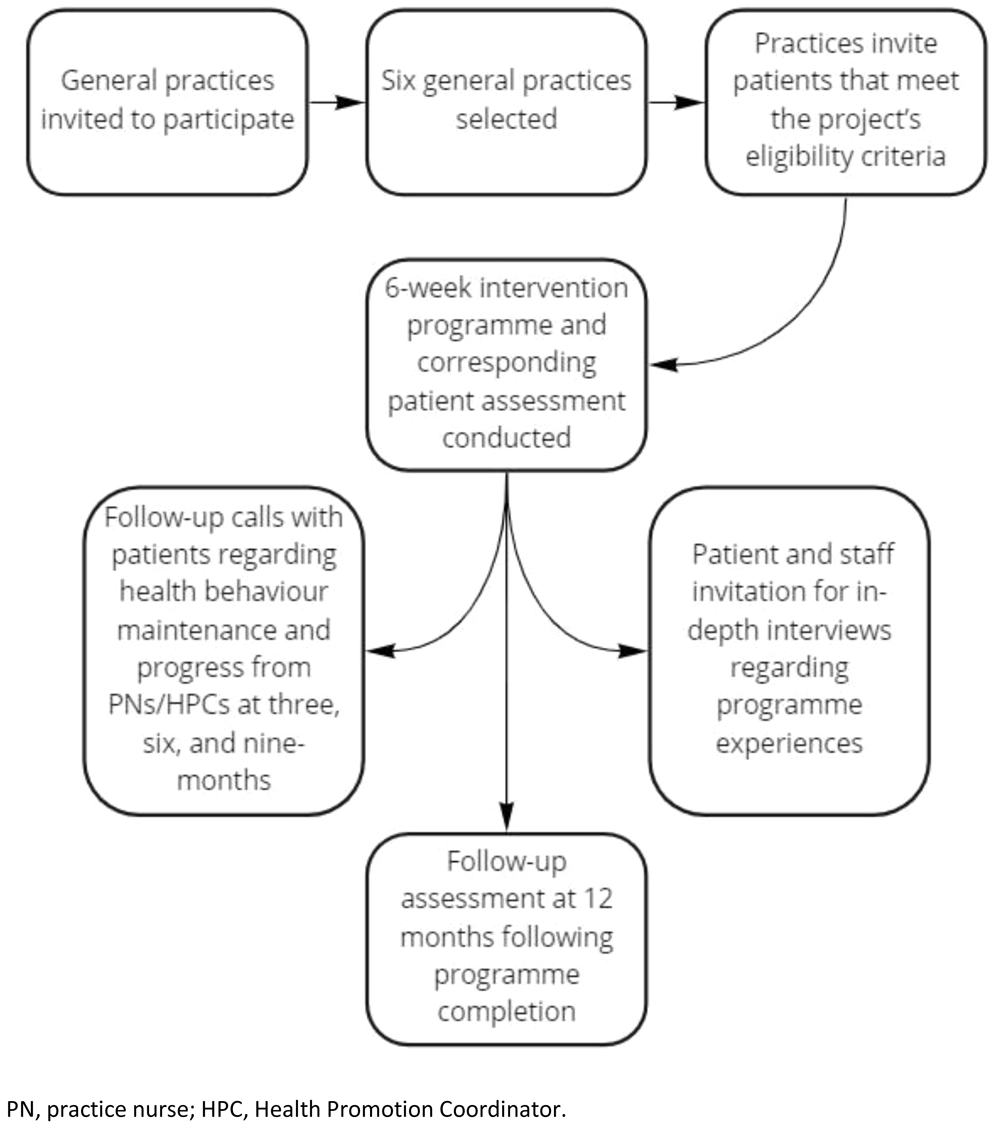
PN, practice nurse; HPC, Health Promotion Coordinator.
Dutiful record keeping by care providers delivering the intervention, and regular engagement between participating patients and care providers, both as part of the study and through usual care provision interactions, will help minimise loss to follow-up, and its implications for data interpretation, in all aspects of the project.
Quantitative evaluation. The intervention’s likely effectiveness will be determined by quantitatively comparing outcomes from the questionnaires administered by PNs and HPCs at Time 1 and Time 2. These assessments encompass questions designed by the project team as well as standardised instruments, and they specifically involve examination of patients’ demographics (e.g., age, gender, country of birth), physical health (e.g., Body Mass Index (BMI), blood pressure, cholesterol, Q-RISK3 scores), self-reported health behaviours (e.g., exercise and dietary habits), mental health, health knowledge (e.g., knowledge of health risk factors and environmental health facilitators/barriers), and motivation to change health behaviours (see Table 1). More details regarding the instrument are available in Table 1.
| Number | Characteristics | Items |
|---|---|---|
| 1 | Demographics | • Age • Gender • Country of birth • Marriage status • Education level • Employment status |
| 2 | Physical health | • Height • Weight • BMI • Waist circumference • Blood pressure • On blood pressure treatment • Resting heart rate • Blood glucose • Cholesterol (LDL, HDL, Triglycerides) • Q-risk scores (i.e., Score of a healthy person the same age, Relative risk, Healthy heart age) |
| 3 | Health behaviour | • Alcohol use9 • Physical activity levels10 • Sedentary behaviour11 • Smoking • Medication adherence • Dietary habits |
| 4 | Mental health | • Mental Health and wellbeing12 |
| 5 | Health knowledge | • CVD / Stoke risk factors13 • Environmental barriers / facilitators14 |
| 6 | Behaviour change | • Willingness to change |
In addition, quantitative data outlining the extent of patient engagement with the programme will also be collected. This data will specifically include metrics regarding the following:
Number of practices participating in the programme.
Number of patients recruited per practice.
Number (%) of patients who completed the six-week programme.
Number (%) of patients who completed follow-up at 3, 6, 9, and 12 months following completion of the six-week programme.
Qualitative assessment. The project’s qualitative component meanwhile involves conducting in-depth semi-structured interviews with participating patients and healthcare providers (e.g., GPs, PNs, HPCs, practice staff, other primary care team members) to examine their experiences of the programme, whether they have been involved in similar initiatives and whether they feel the programme could potentially have positive implications for the health of people in their communities. Interviews are being conducted remotely via telephone with purposefully selected participants on an ongoing basis, recorded with consent, and will be, transcribed, and anonymized prior to analysis and dissemination. The conduct, analysis, and reporting of the project’s qualitative component will be informed by the Standards for reporting qualitative research: a synthesis of recommendations (SRQR) guidelines15.
Environmental considerations. The relationship between environmental influences and population health in participating practices’ communities will be studied by examining the quality of walking routes in practices’ communities, and health facilitators and barriers in local areas such as the availability of green and blue spaces, housing density, population density, fast-food restaurants/takeaways, bars, and off-licences. This information may subsequently inform practice level interventions around health-related geographical planning and will be acquired via existing data sources where available.
Data analysis. Quantitative data will be analysed using a combination of descriptive and inferential statistical methods where appropriate, and analyses will be run using SPSS v26 statistical analysis software (IBM SPSS Statistics, RRID:SCR_016479). Analyses examining sub-groups of interest (e.g., patients assigned to the PN or HPC arms, individual general practices, practice type (urban / rural / mixed urban – rural), patient demographics (e.g., age & gender groups)) will be conducted using suitable statistical tests where appropriate. Missing data will be manged using data imputation and / or missing data analysis techniques as required. Qualitative data will be analysed using Braun and Clarke’s ‘Thematic Analysis’ approach (2008)16. The process of thematic analysis is concerned with basic to advanced encoding of data, subsequently developed into themes. Coding will be conducted using NVivo v12 software (NVivo, RRID:SCR_014802).
The project will transfer project findings to target end-users to maximise uptake and impact on policy, practice, and society. Activities will include targeted transfer activities to policy, science, and wider society and this will be done through policy briefings, scientific manuscripts, workshops for stakeholders. This project will complement work which has been conducted in recent years examining other aspects of the implementation of Ireland’s Primary Care Strategy, e.g., team- and inter-agency working17,18. Data will be destroyed by project investigators after a retention period of three years following study completion to facilitate dissemination activities
Informed consent. All potential participants (i.e., healthcare professionals and patients) must provide informed consent to take part in the project. Informed consent is provided by way of potential participants reading detailed project information leaflets and thereafter signing and returning consent forms to the dedicated research team members. With respect to obtaining patient consent, participating GPs, or a member of their clinical team first contact potential patient participants via existing face to face healthcare appointments, phone call, and text message methods. Interested patients, or their healthcare professional acting on their behalf, can thereafter contact the research team to receive further information on the study and a consent form that patients must sign and return to the research team if they would like to take part. Potential participants are given a “cooling off period” to consider participating in the study and they are informed that their decision will not have any impact on their care. The project was approved by the UCD Human Research Ethics Committee on March 5th, 2021 (LS-20-19-Cullen).
Participant confidentiality/anonymity. Participants must agree, either themselves or through their GP, to provide the researchers with access to their personal details. Alphanumeric codes will be allocated to individual participants to ensure confidentiality / anonymity, and data will only be presented in aggregated form in published project manuscripts, reports, and presentations. Participants can withdraw from the study at any time until their data has been anonymised as at that point their individual data cannot be identified for deletion.
Data protection. Study participants’ personal data cannot be accessed by anyone other than internal study investigators. Electronic data is stored using pseudonymised codes for individual participants on password protected computers at the UCD School of Medicine’s Catherine McCauley Education & Research Centre, Nelson St, Dublin 7, at the Irish Heart Foundation offices at 17-19 Rathmines Road Lower, Dublin 6, and at participating practices. The principal investigator will destroy the data after three years of study completion to facilitate secondary data analysis activities and project dissemination. If study datasets are published online in a data repository, participant identities will be secured using necessary statistical disclosure control techniques.
Vulnerable participants. The study involves engaging with and collecting data from vulnerable populations, most notably persons living in socioeconomically deprived areas and persons at risk of CVD. Nonetheless, the study is deemed minimal risk because it involves secondary data collection, healthcare provider administered assessment, and remote / anonymous data collection via survey and qualitative interview research techniques. All patient participants in the study are judged to be physically and psychologically well enough to take part, and they all explicitly consent to participate. Study information sheets and consent forms include statements noting that patients with concerns should contact their personal GP. Appropriate follow-up with patients’ GPs will be scheduled should study researchers identify potential health concerns among participating patients during their engagements with them.
At the time of writing, the HRPP initiative is nearing completion of:
- The six-week behaviour change programmes at the participating practices.
- Data collection regarding baseline assessments that are conducted prior to patients’ commencement of the six-week behaviour change programme.
- In-depth qualitative interviews with participating HRPP patients and healthcare professionals regarding their experiences and views of the initiative.
- Commencement of 12-month follow-up assessments will be begin shortly.
This protocol outlined our plans to evaluate the feasibility, acceptability, and likely effectiveness of the HSE / Irish Heart Foundation / UCD led HRPP initiative that is being conducted across six general practices in the east of Ireland. The details included in this protocol highlight the necessity for CVD prevention initiatives such as the HRRP programme in communities, the context from which the initiative developed, the actions proposed and taken thus far by clinical and research teams in the conduct of the programme’s delivery, evaluation, and dissemination, ethical considerations, and the status of the project’s ongoing development.
The project aligns with calls within research circles for implementation and evaluation of CVD prevention initiatives that are attuned to the socially and culturally specific care needs of local communities4. Further, the project also represents an important step forward for the Irish healthcare system with regards to advancing knowledge that may inform the development of long-term plans for the routine delivery of more intensive CVD prevention services within community health settings.
The findings of the HRPP evaluation have the potential to offer unique insight into how such CVD prevention initiatives might impact on the long-term health and health related behaviour, knowledge, and attitudes among high-risk patients. Moreover, they may also provide greater perspective on the psychological, logistical, economic, and environmental barriers and facilitators that determine the acceptability of such initiatives among patient and care provider stakeholders. Going forward, the outcomes of this evaluation may inform larger scale interventional research studies in the Irish healthcare context and /or routine care practices within health service settings, and healthcare policy more broadly.
Zenodo: Preventing cardiovascular disease in at-risk patients: Protocol for a feasibility study in general practice ('High-Risk Prevention Programme') - Extended Data. https://doi.org/10.5281/zenodo.64777948.
This project contains the following extended data:
- Consent form_Interview - patients.pdf
- Consent form_Interviews_staff.pdf
- CONSORT extension for Pilot and Feasibility Trials Checklist.pdf
- Information sheet and Consent form_Questionnaire.pdf
- Interview schedule staff.pdf
- Interview schedule_patients.pdf
- Questionnaire instrument.xlsx
- STROBE_checklist_v4_combined.pdf
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Is the rationale for, and objectives of, the study clearly described?
Partly
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Partly
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Intervention development, implementation science, feasibility studies, qualitative research
Is the rationale for, and objectives of, the study clearly described?
Partly
Is the study design appropriate for the research question?
Partly
Are sufficient details of the methods provided to allow replication by others?
Partly
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cardiology
Is the rationale for, and objectives of, the study clearly described?
Partly
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Partly
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Secondary prevention of cardiovascular disease; clinical epidemiology; cardiovascular risk assessment
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 06 May 22 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with HRB Open Research
Already registered? Sign in
Submission to HRB Open Research is open to all HRB grantholders or people working on a HRB-funded/co-funded grant on or since 1 January 2017. Sign up for information about developments, publishing and publications from HRB Open Research.
We'll keep you updated on any major new updates to HRB Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)