Keywords
amyotrophic lateral sclerosis, ALS, respiratory function, C9orf72, disease progression, prognosis
amyotrophic lateral sclerosis, ALS, respiratory function, C9orf72, disease progression, prognosis
The C9orf72 hexanucleotide repeat expansion has been causally linked to amyotrophic lateral sclerosis (ALS)1,2 and frontotemporal dementia (FTD)3. The C9orf72 expansion accounts for up to 10% of those with ALS and 25% of FTD in populations of northern European extraction4. It is associated with a number of distinctive features clinically, namely, earlier disease onset, cognitive and behavioural impairment, distinct neuroimaging changes, family history of neurodegeneration, and decreased survival relative to patients lacking the C9orf72 expansion5–12. In 2016 we observed across five European cohorts that the negative prognosis associated with carriage of the C9orf72 expansion is most pronounced in male patients with spinal onset ALS13.
Decline in respiratory function is one of the most serious symptoms of ALS and respiratory failure is the primary cause of death in most cases; therefore, there is much interest in accurate measurement of respiratory function. We have characterised the longitudinal respiratory decline of 797 ALS and 39 primary lateral sclerosis (PLS) patients from Ireland using the occluded sniff nasal inspiratory pressure (SNIP) respiratory strength measure14. The SNIP is a widely used tool that correlates well with diaphragmatic strength and is considered reliable and reproducible in ALS patients15.
Recently, it has been reported that the C9orf72 repeat expansion is associated with an accelerated deterioration of respiratory function and survival in a cohort of 372 Portuguese patients16. Respiratory function was assessed with the ALSFRS-R respiratory sub-score (ALSFRS-Rresp) and the predicted value of forced vital capacity (%FVC). It was found that %FVC declined significantly faster in patients carrying the C9orf72 expansion compared to those without (P = 0.01), while in Cox models, the C9orf72 expansion was associated with poorer survival (P = 0.002)16. The ALSFRS-Rresp score was not found to be associated with C9orf72.
In this study, we aim to confirm the association of C9orf72 with accelerated respiratory decline and prognosis in an Irish ALS cohort using joint longitudinal and time to event models (referred to as joint models for the rest of this manuscript). Furthermore, we aim to explore respiratory function in C9orf72 by gender and by site of onset subgroups analogous to those we previously characterised to have differential associations with survival in male spinal onset ALS patients13.
The Irish ALS Register complies with the Irish Data Protection Acts 1988-2018 and has been approved by the Beaumont Hospital Ethics Committee (05/49). Written consent, or in cases where the disease process has affected the patients ability to write, oral consent, is obtained from all participants for inclusion on the Irish ALS register and participation in research and written documentation of consent is kept on file at the Academic Unit of Neurology, Trinity College Dublin.
This study includes all patients with a diagnosis according to the El-Escorial criteria of spinal or bulbar onset ALS and who attended the multidisciplinary ALS clinic in Beaumont Hospital, Dublin between 01/01/2001 and 01/12/2018. The population was further limited to those who underwent respiratory assessment, and who had testing for the C9orf72 expansion, performed using repeat-primed PCR, with a cut-off of 30 hexanucleotide repeats or above used to categorise samples as positive for the repeat expansion1. The diagnosis was confirmed by the consultant neurologist (OH) and Riluzole 50mg twice daily was routinely prescribed. Patients provided informed consent for demographic and clinical data including ALSFRS-R and respiratory measurement to be recorded on the Irish ALS register. Patient’s ALSFRS-R scores were evaluated by assessors trained and certified using ENCALS standard operating procedures (ENCALS 2015). Respiratory measurement was via occluded SNIP measurement, which we described in full previously14. Briefly, the preferred nostril was chosen and a nasal probe fitted. Standardized verbal instructions were provided by a trained physiotherapist and each patient completed at least 10 consecutive maximal SNIPs with the contralateral nostril occluded. The highest value for each SNIP method was recorded in cmH20. Follow-up of survival status was through the regular operation of the Irish ALS register, which is carried out on a continuous basis through multiple sources including the General Register Office, www.rip.ie, and family notification of the MDT clinic staff and the IMNDA. For the current analysis, survival status was last updated at the time of data extraction from the register on 07/12/2018.
Longitudinal models of occluded SNIP measurements were constructed as linear mixed effects multi-level models. Follow-up of cases was limited to six years for the purposes of statistical modelling as few patients (2%) survived longer than this time. Time since disease onset was included with random effects per individual and a grouped fixed effect, and occluded SNIP measurements were specified as an interaction with time. A binary term to indicate C9orf72 expansion status was also included, interacting with time and SNIP measurements. Splines were used to allow for non-linear trend of occluded SNIP measurements over time. A delayed entry Cox proportional hazards survival model was constructed, including the important prognostic variables age at onset, diagnostic delay, bulbar onset and C9orf72 status. The longitudinal and Cox models were then used to construct a joint model using the R package JMBayes 0.8-8317. Next, a joint model with the same explanatory variables but with the ALSFRS-Rresp as the dependent longitudinal variable was fitted for those participants with ALSFRS-R data. Finally, to explore the longitudinal trend of occluded SNIP measurements by C9orf72 status in gender and site of onset subgroups, the longitudinal model of occluded SNIP was expanded to include full interaction of the C9orf72 status, gender, site of onset and time variables, before inclusion in a new joint model. Graphs of predicted SNIP were generated from models to visualise the fitted group trends. All statistical analyses were carried out using R Statistical Software version 3.5.118 with additional packages17,19–24. The analysis code is provided (see Software availability).
In this study, 630 ALS patients with a total of 2,165 longitudinal SNIP measurements were included, of whom 58 (9.2%) carried the C9orf72 repeat expansion. Those carrying the C9orf72 expansion were younger (median 56.6 years) when compared to those without the expansion (median 62.0 years) but were similar in other characteristics (Table 1). Comparison via likelihood ratio test of initial linear mixed models of C9orf72 status versus time indicated that inclusion of spline terms improved fit (p < 0.001). Table 2 displays the hazard ratios (HRs) from the Cox proportional hazards model used for the event component of the joint model with age at onset, diagnostic delay, bulbar onset and carriage of the C9orf72 expansion; all prognostic in the Cox model.
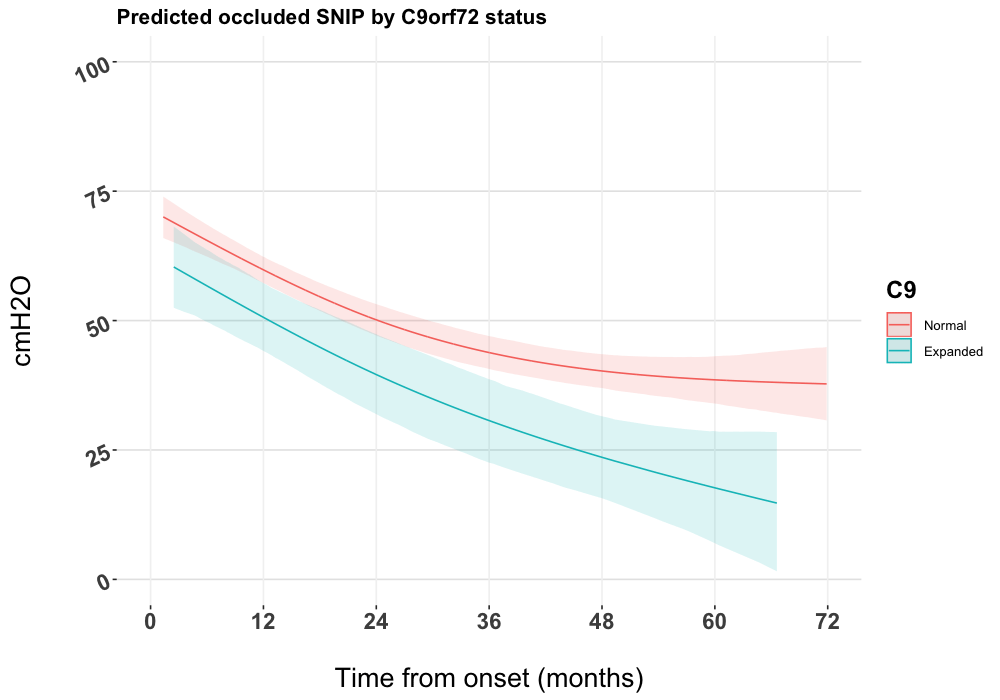
Figure 1 displays the longitudinal characteristics of the occluded SNIP measurements by C9orf72 expansion status for all patients as modelled via joint modelling. Patients without the C9orf72 expansion had, on average, higher scores than those carrying the C9orf72 expansion across the complete follow-up time. The difference between groups was greater over time, particularly after three years from disease onset. Table 2 displays the hazard ratios for the Cox proportional hazards model and for the posterior estimated HRs of the survival sub-model of the joint model.
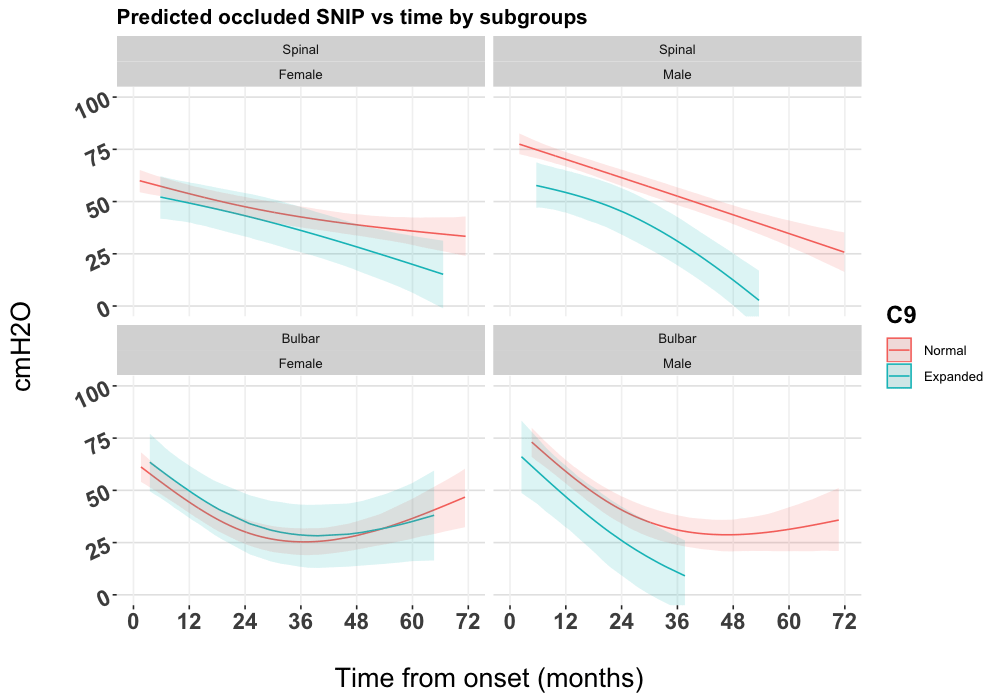
The exploratory model including full interaction between time, C9orf72 status, site of onset and gender was used to generate Figure 2. The deviance information criterion (DIC) indicated a better fit for the model with interaction between time, C9orf72 status, site of onset and gender (DIC: 42,485) than the model including C9orf72 status only (DIC: 42,646). Figure 2 shows distinct curves between C9orf72 normal patients and C9orf72 expanded patients in males only, while in females the trends are virtually indistinguishable. Among males there appears to be a greater distinction by C9orf72 status in spinal onset patients than in bulbar onset patients.
Of the 630 patients included in the study, only 450 had a total of 1,728 contemporaneous SNIP and ALSFRS-R_resp measurements. Figure 3 displays the longitudinal characteristics of the ALSFRS-R_resp by C9orf72 expansion status. In contrast to Figure 1, Figure 3 shows that the ALSFRS-R_resp is indistinguishable between C9orf72 normal and C9orf72 expanded patients in the earlier years of the disease course. After approximately three years, the trends begin to diverge; however, the credible intervals remain overlapping.
In this study we confirmed the findings of Miltenberger-Miltenyi et al.16 that carriage of the C9orf72 expansion in ALS is associated with both survival and an accelerated decline in respiratory function in comparison to ALS without the expansion. We also found that the ALSFRS-R_resp did not differentiate rate of decline by C9orf72 status within the first three years, as shown by direct respiratory strength testing using occluded SNIP. This confirms the similar finding using %FVC to measure respiratory function by Miltenberger-Miltenyi et al.16. Our analysis differs from that of Miltenberger-Miltenyi et al.16 through the use of splines to allow for non-linear trends for SNIP and ALSFRS-R_resp decline over time, and the use of joint models to account for differential loss to follow-up and estimation of the effect of the longitudinal terms in the survival sub-model. Additionally, our exploratory model demonstrated that the association between respiratory function and C9orf72 status is more distinct in male patients, particularly in those with spinal onset disease, which may explain our previous observations in European populations of a worse prognosis in male spinal onset C9orf72 expansion carrying patients13.
The comparison of hazard ratios from Cox and joint models indicates that after controlling for longitudinal SNIP measurements, the bulbar onset and presence of the C9orf72 expansion are no longer strongly associated with the risk of death, and that the SNIP measurement itself is predictive of survival. These results are suggestive that respiratory strength decline may explain part of the survival effect of the C9orf72 expansion in ALS. Alternatively, another characteristic of C9orf72 expansion ALS, such as cognitive or behavioural dysfunction, may mediate this relationship5,12,25. Conversely, it is plausible that both disease subtypes follow a common path towards respiratory dysfunction after some earlier biochemical convergence, with the C9orf72 expanded group having reached that point more rapidly - i.e. the findings may reflect a faster overall disease process rather than faster progress in respiratory function alone. Therefore, while Miltenberger-Miltenyi et al. hypothesise that a pathophysiological link between C9orf72 and respiratory function may occur due to an interaction between disordered regulation of the homeobox gene (Hoxa5) and C9orf72 mutated proteins16,26, such direct interaction between C9orf72 and respiratory function may not be required to explain these results.
Our finding that the ALSFRS-R_resp did not differentiate between C9orf72 normal and C9orf72 expanded ALS is congruent with findings that ALSFRS-R_resp questions are not sensitive to the respiratory burden of the majority of patients27–29. Our previous study on longitudinal sub-scores of the ALSFRS-R in ALS cases unstratified by C9orf72 status found that ALSFRS-R_resp had a worse ability to distinguish spinal and bulbar onset disease than bulbar and motor sub-scores did, and additionally had less prognostic value27. In addition, Franchignoni et al. found that the ALSFRS-R_resp questions were subject to frequent ceiling responses and suggested the addition of one or two questions of intermediate difficulty to improve reliability and personal discrimination of this sub-score29. Therefore, our current results suggest that the occluded SNIP could provide a suitable metric with which to augment the ALSFRS-R as an alternative to additional intermediate questions. Furthermore, as joint models provide a framework for combined analysis of longitudinal measurements and survival, they can extend to model multiple longitudinal measurements, and in addition, recent analysis has shown that joint models may provide greater statistical power in ALS trials with functional and mortality outcomes compared to other approaches30.
Our analysis benefits from a large number of longitudinal measurements with up to six years follow-up in a cohort of 630 ALS patients, including 58 who carried the C9orf72 expansion. Even though we used the occluded SNIP as a metric of respiratory function, which differs from the use of %FVC by Miltenberger-Miltenyi et al.16, our results are congruent with theirs. In addition, the use of joint models allowed us to demonstrate the impact of longitudinal respiratory function on survival while at the same time accounting for differential loss to follow-up in longitudinal occluded SNIP measurements. The main limitation is that the analysis did not include longitudinal data on cognitive or behavioural function, which may play an important role in mediating the effects of C9orf72 on survival in ALS.
Our results confirm findings from Portugal that the C9orf72 repeat expansion is associated with both survival and accelerated respiratory function decline in ALS, and that the ALSFRS-R_resp does not differentiate respiratory function between C9orf72 normal and C9orf72 expanded cases in the first three years of follow-up. Furthermore, we demonstrated through the use of joint models that respiratory function measured using occluded SNIP carries prognostic importance and may explain previous observations in European cohorts of a worse prognosis in male spinal onset ALS patients that carry the C9orf72 expansion.
The raw data from this study cannot be sufficiently de-identified, and therefore are not publicly available. As ALS is a rare disease, we are very conscious of protecting privacy of patients. In this particular analysis, low numbers of cases at certain age ranges mean we could not guarantee privacy if we were to publish the data in full. However, the data from the current study are available for further research purposes on reasonable request. To access the data, please contact the Principal Investigator (orla@hardiman.net). Researchers must provide a written proposal on how the data will be used in research before access is granted.
Source code available from: https://github.com/jpkrooney/ALS_C9orf72_Resp_function_Paper
Archived source code at time of publication: https://doi.org/10.5281/zenodo.3445433
License: GPL3
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Amyotrophic Lateral Sclerosis.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Pinto S, Alves P, Pimentel B, Swash M, et al.: Ultrasound for assessment of diaphragm in ALS.Clin Neurophysiol. 2016; 127 (1): 892-897 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical genetics, neurology.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 26 Sep 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with HRB Open Research
Already registered? Sign in
Submission to HRB Open Research is open to all HRB grantholders or people working on a HRB-funded/co-funded grant on or since 1 January 2017. Sign up for information about developments, publishing and publications from HRB Open Research.
We'll keep you updated on any major new updates to HRB Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)